Pressure P, volume V and temperature T for a certain gas are related by P=AT−BT2V, where A and B are constatns .The work done by the gas as its temperature change from T1 to T2 while pressure remaining constatn is
A−B2(T2−T1)
A(T2−T1)−B(T22−T21)
AT(T22−T21)−B3(T32−T31)
A(T2−T1)2−B3(T2−T1)3
The correct Answer is:B
p=AT−BT2V or V=AT−BT2p
∴dV=AdT−B×2TdTP, since p is constant
or pdv=(A−BT)dT
W=∫T2T1pdV=∫T2T1(A−2BT)dT⇒A(T2−T1)−B(T22−T21)
Similar Questions
Pressure p, volume V and temperature T for a certain gas are related by
p=αT−βT2V
where, α and β are constants. Find the work done by the gas if the temperature changes from T1 to T2 while the pressure remains the constant.
View SolutionFor a thermodynamic system the pressure, volume and temperature are related as new gas law given as
P=αT2V
Here α is a constant. Find the work done by the system in this process when pressure remains constant and its temperature changes from T0 to 2T0.
View Solution
Knowledge Check
Pressure P, voluume V and temperature T for a certain gas are related by P=AT−BT2V where A and B are constant. The work done by the gas as its temperature change from T1 to T2 with pressure remaining constant is
AA−B2(T2−T1)BA(T2−T1)−B(T22−T21)CA2(T22−T21)−B3(T32−T33)DA(T2−T1)2−B3(T2−T1)3Pressure P, volume V and temperature T for a certain gas are related by P=AT−BT2V, where A and B are constants .The work done by the gas as its temperature change from T1 to T2 while pressure remaining constant is
AA−B2(T2−T1)BA(T2−T1)−B(T22−T21)CA2(T22−T21)−B3(T32−T31)DA(T2−T1)2=B3(T2−T1)3Pressure P, Volume V and temperature T of a certain material are related by the P=αT2V. Here α is constant. Work done by the material when temparature changes from T0 to 2T0 while pressure remains constant is :
A6αT30B32αT30C2αT20D3αT20
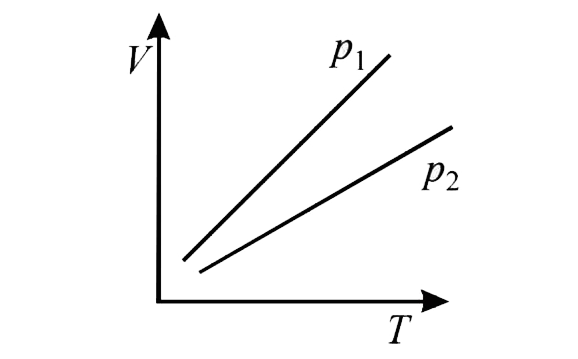
The volume V versus temperature T graphs for a cetain amount of a perfect gas at two pressure p1andp2 are as shown in Fig. It follows from the graphs that p1 is greater than p2.
View SolutionPressure P, volume V and temperature T of a certain material are related by P=αT2/V where α is contant. Work done by the material when temperature changes from T0 to 2T0 and pressure remains constant is
View SolutionOne mole of an ideal gas at temperature T1 expends according to the law PV2=a (constant). The work done by the gas till tempereature of gas becomes T2 is
View SolutionThe equation of state for a real gas having pressure P, volume V and temperature T is expressed as
(P+aV2)(V−b)=RT
where a, b and R are constant. What are the dimensional formula of ab?
View SolutionOne mole of an ideal gas at temperature T expands slowly according to the law pV= constant.
Its final temperature is T2. The work done by the gas is
View Solution
