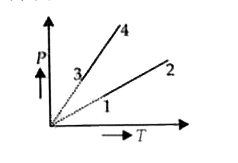
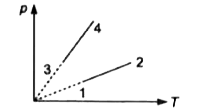
Pressure versus temperature graph of an ideal gas of equal number of moles of different volumes is plotted as shown in Fig. Choose the correct alternative.
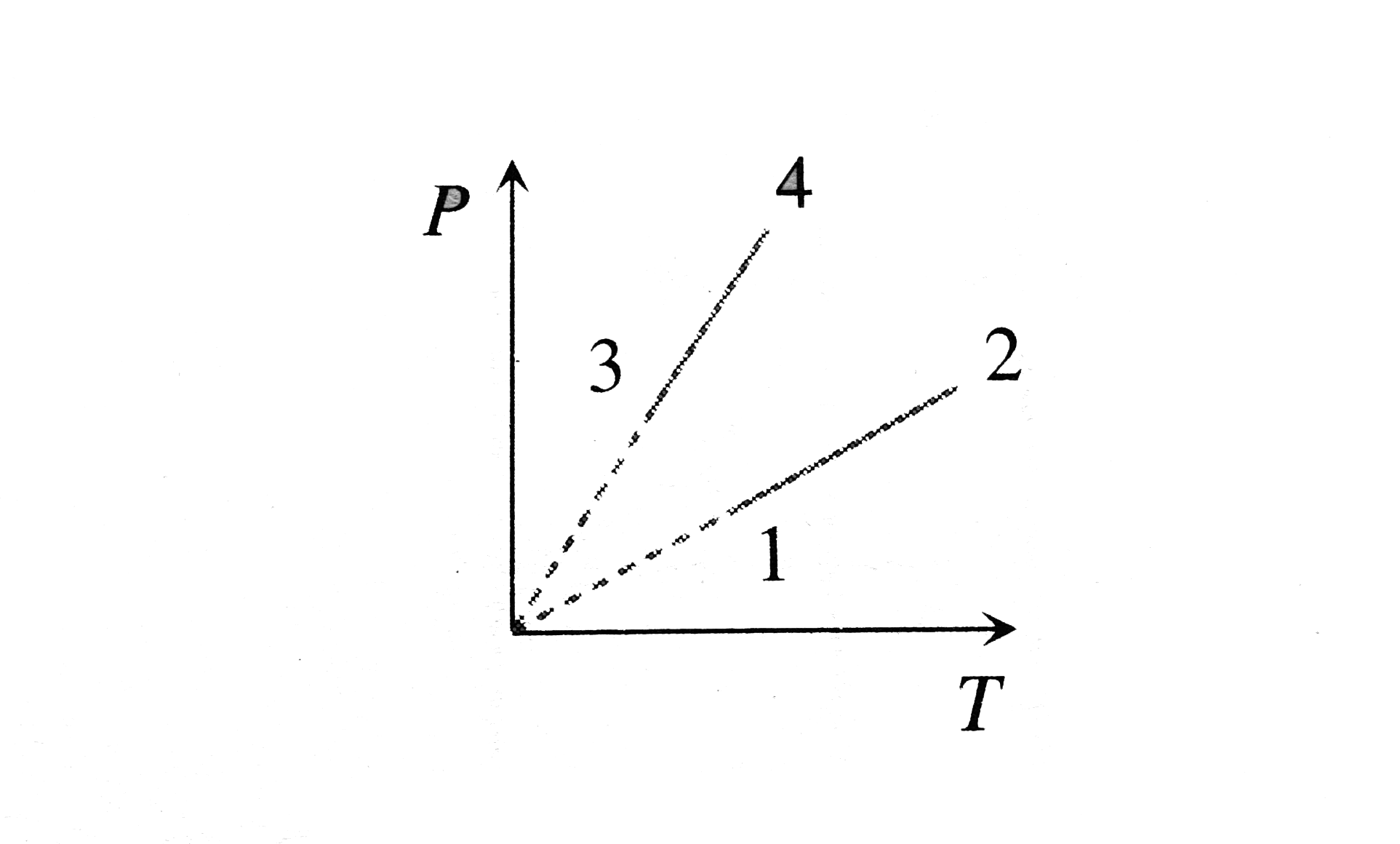
V1=V2=V3=V4
V4>V3>V2>V1
V1=V2,V3=V4andV2>V3
V1=V2,V3=V4andV2<V3
The correct Answer is:C
As P-T graphs are straight lines, passing through origin, therefore, P∝T. But as P=(μRV)T.
thererore, the slope of straight line ∝1V
As(Slope)12<(Slope)34 ∴V2>V3
As for a given slope, V is constant, therefore,
V1=V2andV3=V4.
Similar Questions
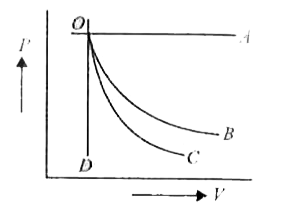
A graph of pressure versus volume for an ideal gas for different process is as shown. In the graph curve OC represents
View SolutionPressure versus temperature graph of an ideal gas of equal number of moles of different volumes is plotted as shown in Fig. Choose the correct alternative.

View Solutionकिसी आदर्श गैस के विभिन्न आयतनों के मोलों की समान संख्या के दाब-ताप ग्राफ को चित्र में दिखाए गए अनुसार आरेखित किया गया है। सही विकल्प को चुनिए।
View Solutionचित्र में, आदर्श गैस के भिन्न आयतन परन्तु समान मोलो के लिए ताप-दाब ग्राफ प्रदर्शित है। सही विकल्प चुनिये
View SolutionPressure versus temperature graphs of an ideal gas are as shown in figure. Choose the wrong statement
View SolutionPressure versus temperature graph of an ideal gas at constant volume V is shown by the straight line A. Now mass of the gas is doubled and the volume is halved, then the corresponding pressure versus temperature graph will be shown by the line
View SolutionTemperature versus pressure graph of an ideal gas is shown in Fig. During the process AB
View SolutionPressure versus temperature graph of an ideal gas of equal number of moles of different volumes is plotted as shown in Fig. Choose the correct alternative.
View Solution
