Starting with the same initial conditions, an ideal gas expands from volume V1→V2 in three different ways. The work done by the gas is W_1 if the process is purely isothermal, W2if purely isobaric and W3 if purely adiabatic. Then
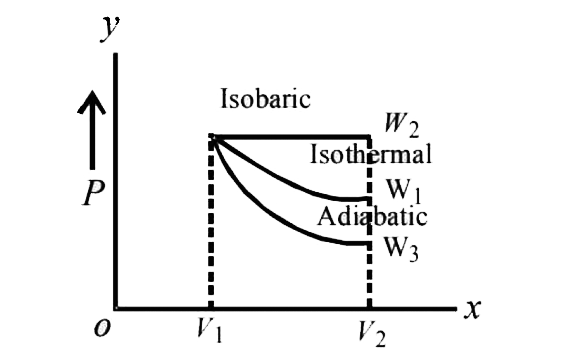
W2>W1>W3
W2>W3>W1
W1>W2>W3
W1>W3>W2
The correct Answer is:a
Step by step video, text & image solution for Starting with the same initial conditions, an ideal gas expands from volume V_1 to V_2 in three different ways. The work done by the gas is W_1 if the process is purely isothermal, W_2if purely isobaric and W_3 if purely adiabatic. Then by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- IIT QUESTIONS 1Book:D MUKHERJEEChapter:IIT QUESTIONS 1Exercise:Assertion- Reason Type
Explore 2 Videos
- IIT QUESTIONS 1Book:D MUKHERJEEChapter:IIT QUESTIONS 1Exercise:Linked- Comprehension Type
Explore 3 Videos
- HEAT AND THERMODYNAMICSBook:D MUKHERJEEChapter:HEAT AND THERMODYNAMICSExercise:All Questions
Explore 85 Videos
- IIT QUESTIONS 2Book:D MUKHERJEEChapter:IIT QUESTIONS 2Exercise:Matrix matching type
Explore 1 Video
Similar Questions
STATEMENT-l : In an elastic collision between two bodies, the relative speed of the bodies after collision is equal to the relative speed before the collision.
STATEMENT-2 : In an elastic collision, the linear momentum of the system is conserved.
View SolutionTwo discs A and B are mounted coaxially ona vertical axle. The discs have moments of inertia l and 2l respectively about the common axis. Disc A is imparted an initial angular velocity 2ω using the centre potential energy of a spring compressed by a distance x1. Disc B is imparted angular velocity ω by a spring having the same spring constant and compressed by a distance x2. Both the disc rotate in the clockwise direction.
The rotation x1/(x2 is.
View SolutionTwo holes of unequal diameters d1 and d2(d1>d2) are cut in metal sheet is heated
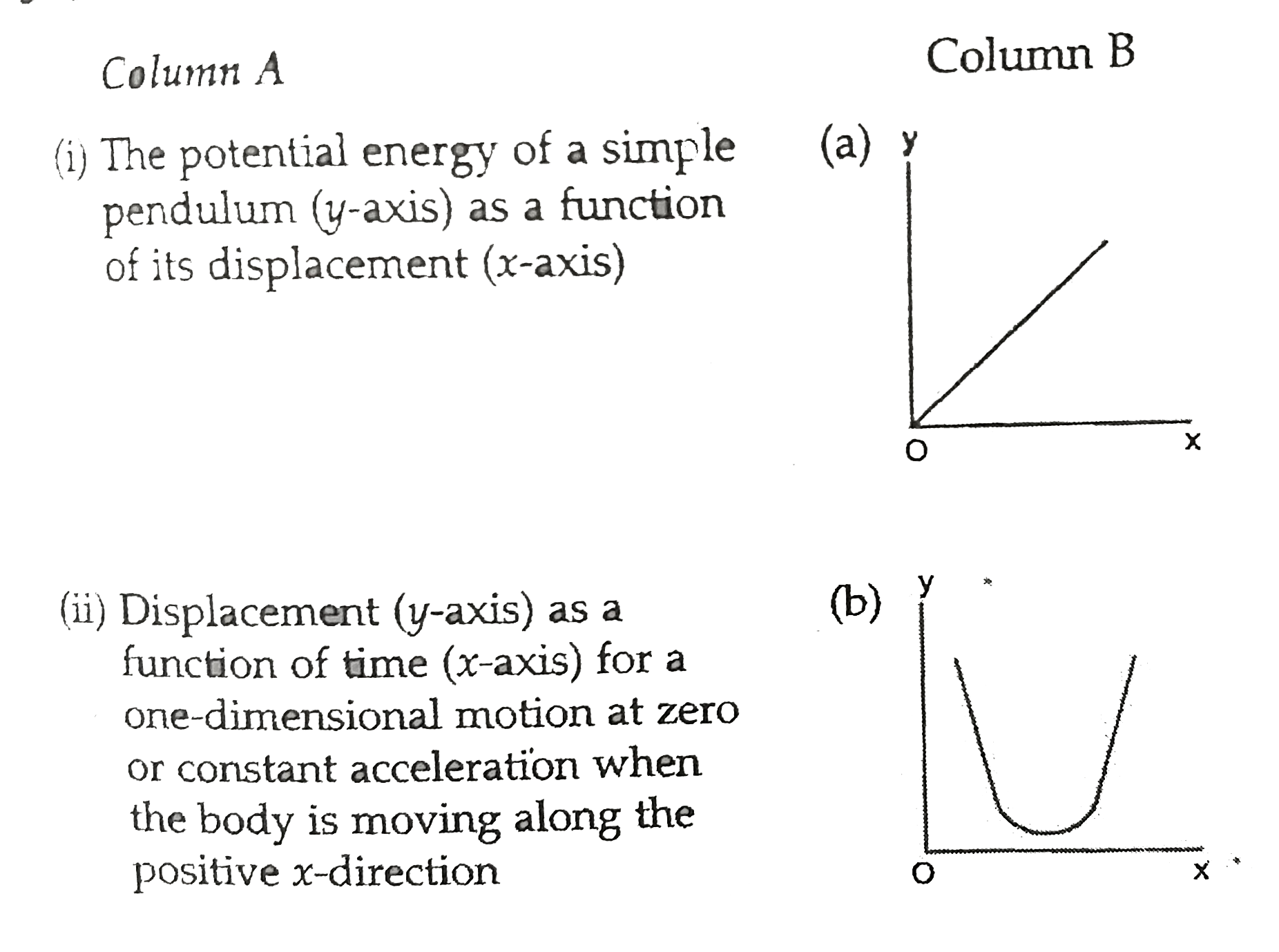
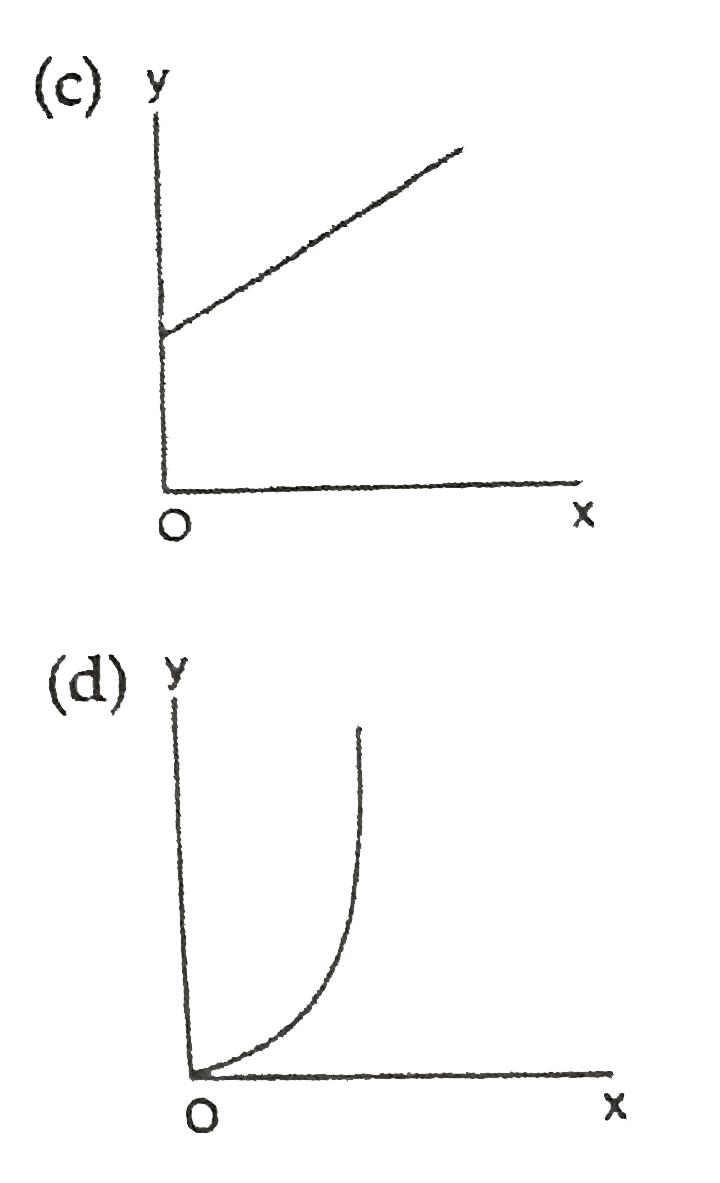
View SolutionColumn A gives a list of possible set of parameters measured in some experiments . The variations of the parameters in the form of graphs are shown in column B.
View SolutionStarting with the same initial conditions, an ideal gas expands from volume V_1 to V_2 in three different ways. The work done by the gas is W_1 if the process is purely isothermal, W_2 if purely isobaric and W_3 if purely adiabatic. Then
View SolutionStarting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways, the work done by the gas is W1 if the process is purely isothermal, W2 if purely isobaric and W3 if purely adiabatic, then
View SolutionWith same initial conditions , an ideal gas expands from volume V1 to V2 in three different ways . The work done by the gas is W1 if the process is isothermal , W2 if isobaric and W3 if adiabatic , then
View SolutionStarting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2 if purely isobaric and W3 if purely adiabatic. Then SS
View SolutionStarting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2 if purely isobaric and W3 is purely adiabatic. Then
View SolutionIf w_1, w_2, w_3 , and we are work done in isothermal adiabatic, isobaric and isochoric reversible processes, then the correct sequence (for expansion) would be
View SolutionThe initial pressure and volume of a gas are P_i and V_i . The gas after expansion attains final volume V_f . Let W_1, W_2 and W_3 are the corresponding work done by the gas, under isothermal, adiabatic and isobaric processes, respectively. Then,
View SolutionIsothermal, isobaric, isochoric and adiabatic processes are some special thermodynamic processes. In which of these processes, the work done is maximum, when a gas expands from V_1 to V_2 ?
View SolutionStarting with same initial conditions, an ideal gas expands from volume V_1 to V_2 in three different ways. The work done by the gas is W_1 if the process is purely isothermal, W_2 if purely isobaric and W_3 if purely adiabatic. Then
View SolutionStarting with the same initial conditions an ideal gas expands from volume V_(1)" to "V_(2) in three different ways. The work done by the gas is W_(1) if the process is purely isothermal, W_(2) if purely isobaric and W_(3) if purely adiabatic. then
View SolutionStarting with the same initial conditions an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is w1 if the process is purely isothermal, W2 if purely isobaric and W3 if purely adiabatic. Then
View SolutionStarting with the same initial conditions, an ideal gas expands from volume V_1 to V_2 in three different ways, the work done by the gas is W_1 if the process is purely isothermal, W_2 if purely isobaric and W_3 if purely adiabatic, then
View Solution
