Temperature of two moles of an ideal gas is increased by 300K in a process V=aT, where a is positive constant. Find work done by the gas in the given process.
VT= constant
or V(pV)=constant (as T=∝pV)
∴pV2= constant
Comparing with pVx= constant, we have
x=2
W=nRΔT1−x
=(2)(R)(300)1−2
=−600R
Similar Questions
Method 1 of Q
Temperature of two moles of a monoatomic gas is increased by 300 K in the process p∝V.
(a) Find molar heat capacity of the gas in the given process.
(b) Find heat given to the gas in that.
View SolutionTemperature of two moles of a monoatomic gas is increased by 300K in the process p∝V. Find
(a) molar heat capacity of the gas in the given process
(b) heat required in the given process.
View Solution
Knowledge Check
The temperature of n moles of an ideal gas is increased from T to 4T through a process for which pressure PaT−1 where a is a constant .Then the work done by the gas is
AnRTB4nRTC2nRTD6nRTThe temperature of n moles of an ideal gas is increased from T to 4T through a process for which pressure P=aT−1 where a is a constant. Then, the work done by the gas is
AnRTB4nRTC2nRTD6nRTTwo moles of an ideal gas are undergone a cyclic process 1-2-3-1. If net heat exchange in the process is 300J, the work done by the gas in the process 2-3 is
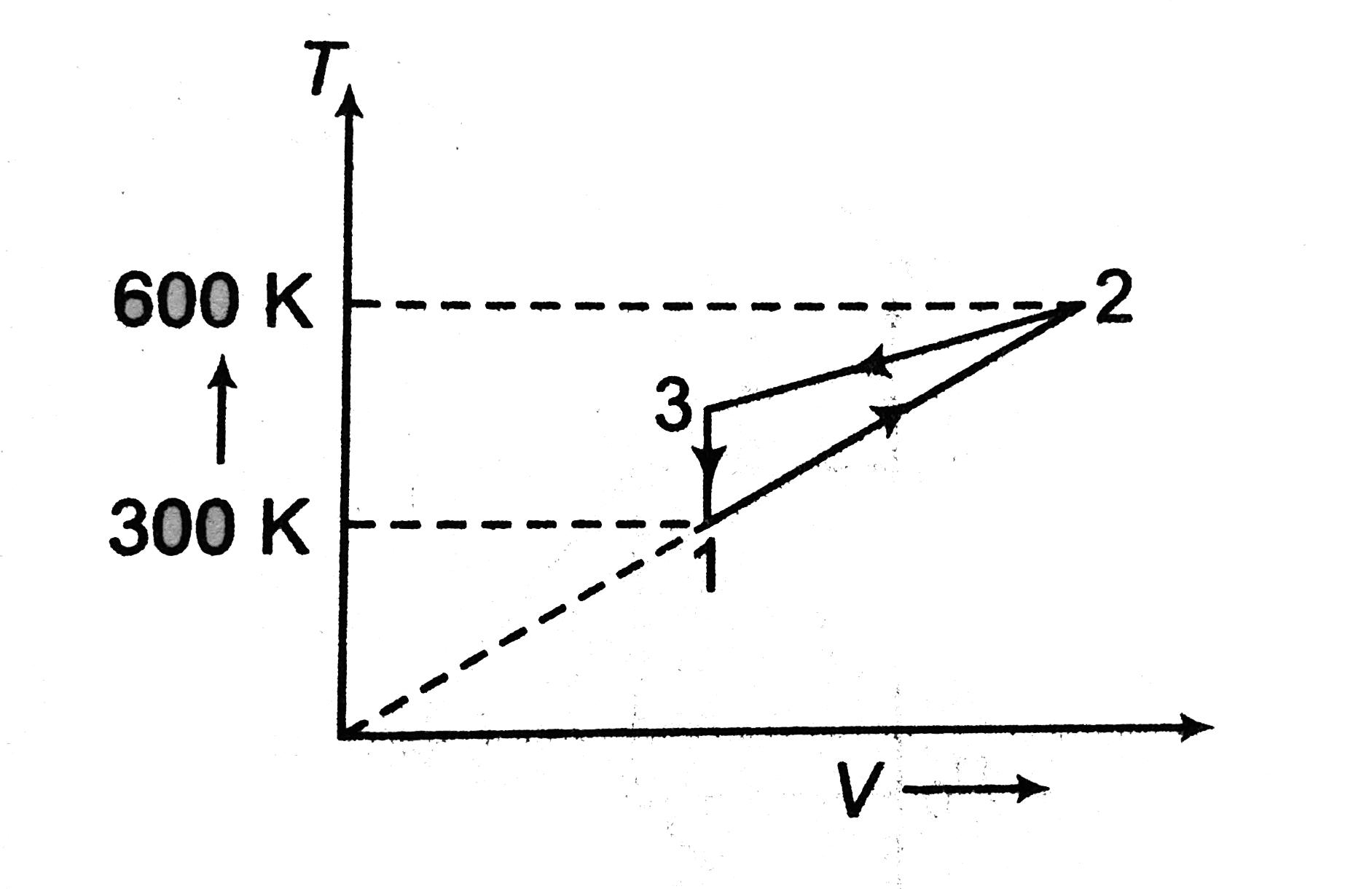 A−500JB−5000JC−3000JDNone of these
A−500JB−5000JC−3000JDNone of these
Temperature of four moles of a monoatomic gas is increased by 300K in isochoric process. Find W, Q and ΔU.
View SolutionOne mole of an ideal gas is heated from 273K to 546K at constant pressure of 1 atmosphere. Calculate the work done by the gas in the process.
View SolutionMethod 4 of W
The temperature of n-moles of an ideal gas is increased from T0 to 2T0 through a process p=αT. Find work done in this process.
View SolutionMethod 1 of ΔU
Temperature of two moles of a monoatomic gas is increased by 600 K in a given process. Find change in internal energy of the gas.
View SolutionTemperature of 1 mole of an ideal gas is increased from 300K to 310K under isochoric process. Heat supplied to the gas in this process is Q=25R, where R=universal gas constant. What amount of work has to be done by the gas if temperature of the gas decreases from 310K to 300K adiabatically?
View Solution
