The absolute temperature of a gas is made 4 time its initial value. What will be the change in rms velocity of its molecules ?
Step by step video solution for The absolute temperature of a gas is made 4 time its initial value. What will be the change in rms velocity of its molecules ? by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
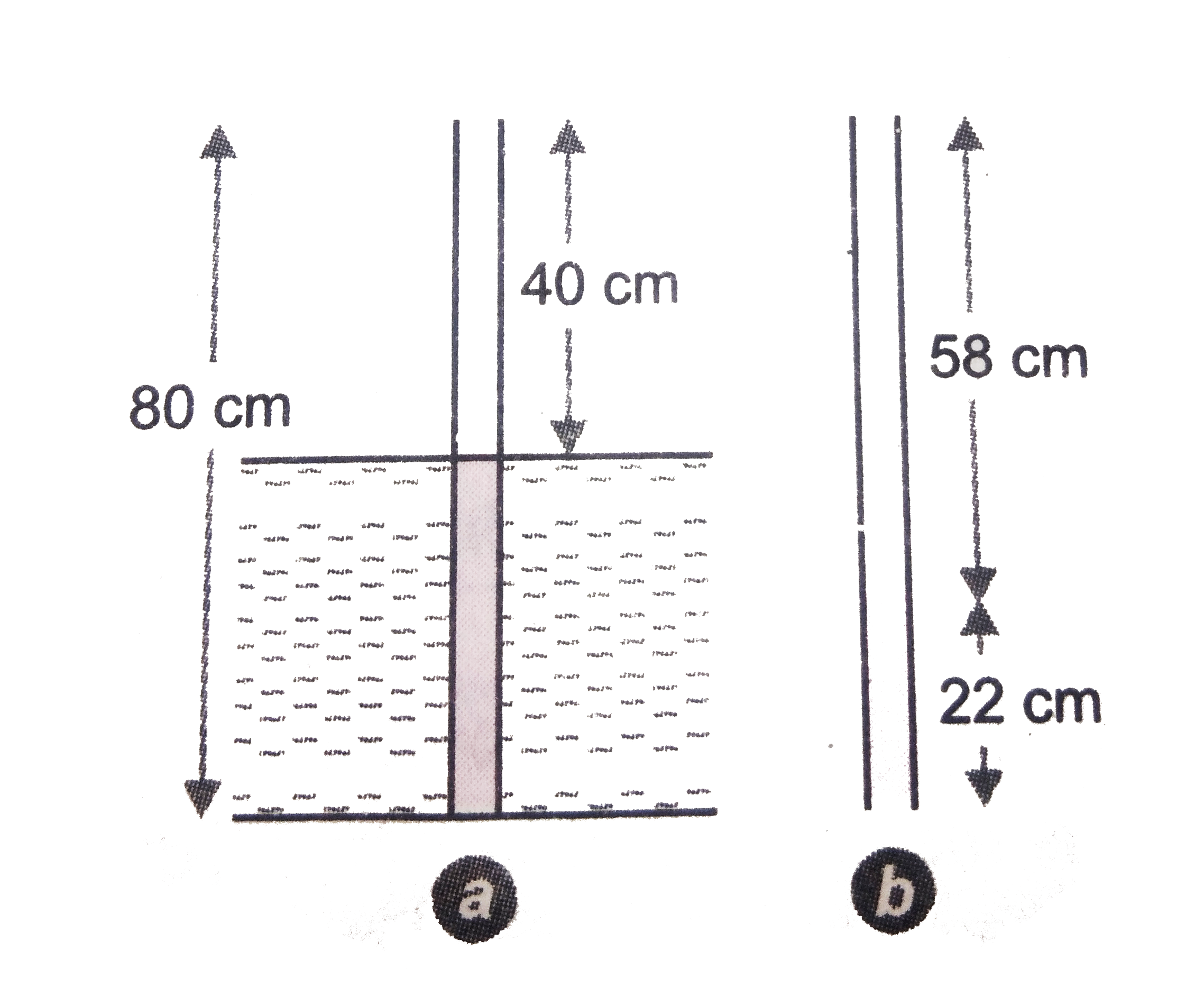
A narrow uniform glass tube 80 cm long and open at both ends is half immersed in mercurry. Then the top of the tube is closed and it is taken out of mercury. A column of mercury 22 cm long then remains in the tube. What is the atmospheric pressure?
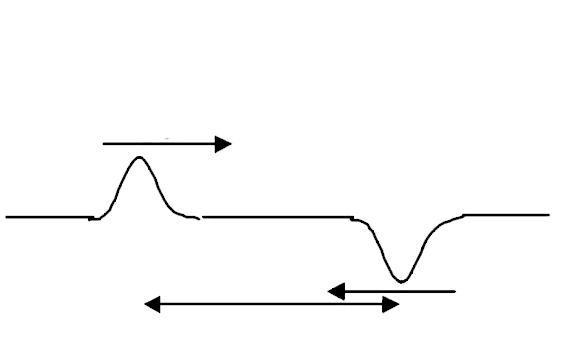
View SolutionTwo pulse in a stretched string whose centers are initially 8cm apart are moving towards each other as shown in the figure. The speed of each pulse is 2cm/s. After 2seconds, the total energy of the pulse will be
View SolutionA force acts for 10 s on a body of mass 10 kg after which the force ceases and the body describes 50 m in the next 5 s. Find the magnitude of the force.
View SolutionThe absolute temperature of a gas is made 4 time its initial value. What will be the change in rms velocity of its molecules ?
View SolutionThe temperature of H_(2) at which the rms velocity of its molecules is seven times the rms velocity of the molecules of nitrogen at 300 K is
View SolutionAt what temperature. The rms velocity of gas molecules would be double of its value at NTP,if pressure is remaining constant?
View SolutionA gas is compressed isothermally . The rms velocity of its molecules
View SolutionThe temperature of H2 at which the rms velocity of its molecules is seven times the rms velocity of the molecules of nitrogen at 300 K is :
View SolutionThe temperature of H_2 at which the rms velocity of its molecules is seven times the rms velocity of the molecules of nitrogen at 300 K is :
View SolutionIF the absolute temperature of a perfect gas rises to four times its initial value estimate the changes of molecular rms speed.
View SolutionIF the absolute temperature of a perfect gas rises to four times its initial value estimate the changes of total kinetic energy.
View SolutionHow will the rms speed and the total kinetic energy of the gas molecules change, if its absolute temperature is decreased 4 times?
View SolutionThe temperature of a gas in increased from 27∘C to 327∘C. Show that the rms velocity of the gas molecules at higher temperature is √2 times the velocity at the initial temperature.
View SolutionThe absolute temperature of a gas is increased 3 times. What will be the incraese in rms velocity of the gas molecule?
View SolutionIf the absolute temperature of a gas is increased 3 times the rms velocity of the molecules will be ............. .
View SolutionIf the absolute temperature of a gas is increased 3 times the rms velocity of the molecules will be ............. .
View Solution

