The efficiency of an ideal gas with adiabatic exponent 'γ' for the shown cyclic process would be
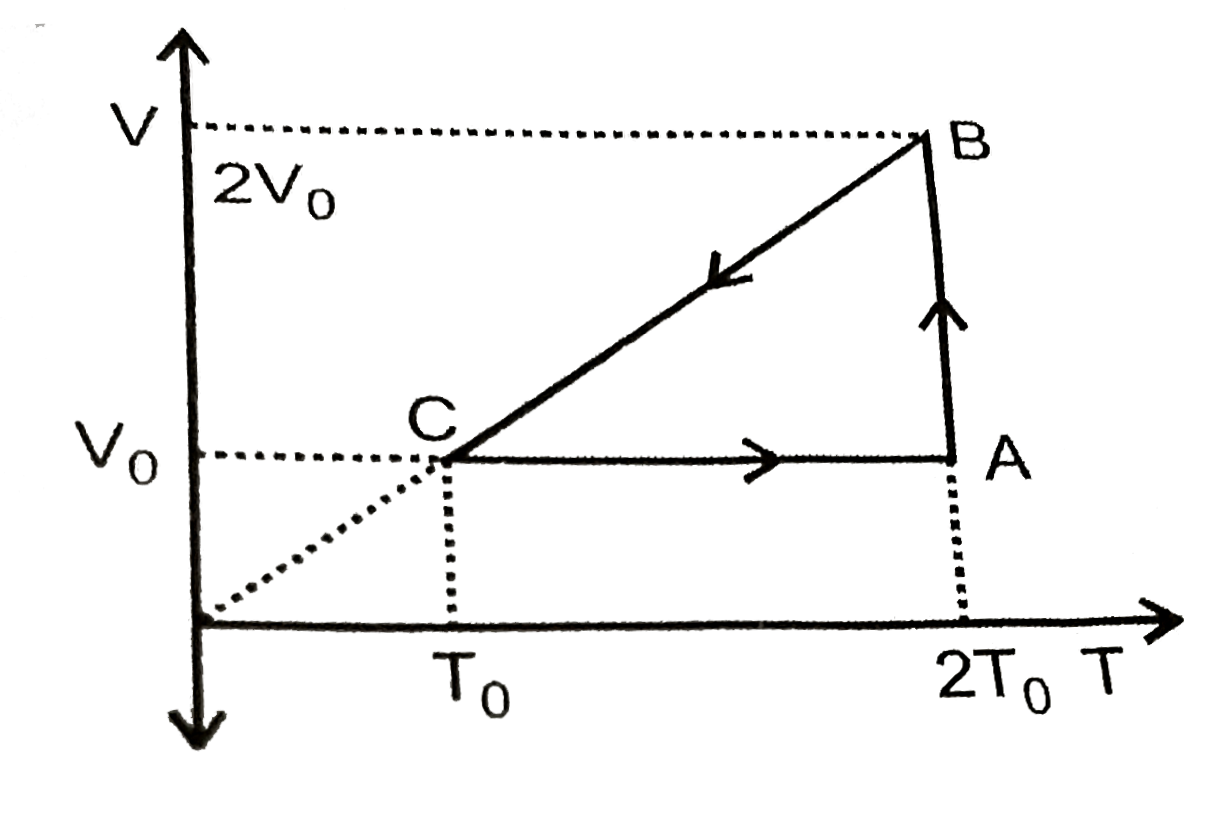
2in2−1γ/(γ−1)
1−1in2γ/(γ−1)
2in2+1γ/(γ−1)
2in2−1γ/(γ+1)
The correct Answer is:A
Step by step video, text & image solution for The efficiency of an ideal gas with adiabatic exponent 'gamma' for the shown cyclic process would be by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLVD PAPERS 2017 NEET, AIIMS & JIPMERBook:DC PANDEYChapter:SOLVD PAPERS 2017 NEET, AIIMS & JIPMERExercise:Solved paper 2018(AIIMS)
Explore 28 Videos
- SIMPLE HARMONIC MOTIONBook:DC PANDEYChapter:SIMPLE HARMONIC MOTIONExercise:Integer type questions
Explore 14 Videos
- SOUND WAVESBook:DC PANDEYChapter:SOUND WAVESExercise:Exercise 19.7
Explore 4 Videos
Similar Questions
Find the molar heat capacity of an ideal gas with adiabatic exponent γ for the polytorpic process PVn= Constant.
View SolutionOne mole of an ideal gas with the adiabatic exponent γ goes through a polytropic process as a result of which the absolute temperature of the gas increases τ−fold. The polytropic constant equal n. Find the entropy increment of the gas in this process.
View Solution
Knowledge Check
Adiabatic exponent of a gas is equal to
ACP×CVBCPCVCCP−CVDCP+CVAn ideal gas with adiabatic exponent (γ=1.5) undergoes a process in which work done by the gas is same as increase in internal energy of the gas. Here R is gas constant. The molar heat capacity C of gas for the process is:
AC=4RBC=0CC=2RDC=RAn ideal gas with adiabatic exponent γ undergoes a process in which internal energy depends on volume
as U=aVα then select the correct statement .AChange in internal energy isγ−1αΔTBMolar heat capacity of process is R1−γ+RαCHeat exchange in the process is given by ΔU[1+1−γα]DEquation of process is given is PVx=constant , where x>0ifα<1.
The volume of one mode of an ideal gas with adiabatic exponent γ is varied according to the law V=a/T, where a is constant . Find the amount of heat obtained by the gas in this process, if the temperature is increased by ΔT.
View SolutionOne mole of an ideal gas with adiabatic exponent γ undergoes the process
(a) P=P0+αV
(b) T=T0+αV
Find Molar heat capacity of the gas as a function of its volume.
View SolutionCalculate the efficiency of a cycle consisting of isothermal, isobaric, and isochoric line, if in the isothermal process the volume of the ideal gas with the adiabatic exponent γ.
(a) increased n−fold ,
(b) decreases n−fold.
View SolutionThe volume of one mole of an ideal gas with adiabatic exponent γ=1.4 is vaned according to law V=a/T where 'a' is a constant. Find amount of heat absorbed by gas in this process if gas temperature increases by 100 K.
View SolutionAn ideal gas with adiabatic exponent gamma is heated isochorically.If it absorbs Q amount heat then, fraction of heat absorbed in increasing the internal energy is
View Solution
