The electron in a hydrogen atom makes a transition from n=n1 to n=n2 state. The time period of the electron in the initial state (n1) is eight times that in the final state (n2). The possible values of n1 and n2 are
n1=8,n2=1
n1=4,n2=2
n1=2,n2=4
n11,n2=8
The correct Answer is:B
To solve the problem, we need to analyze the relationship between the time period of the electron in different energy states of a hydrogen atom.
1. Understanding the Time Period Relation:
The time period T of an electron in a hydrogen atom is related to the principal quantum number n. The time period can be expressed as:
T∝n3
This means that the time period is directly proportional to the cube of the principal quantum number.
2. Setting Up the Equation:
According to the problem, the time period of the electron in the initial state n1 is eight times that in the final state n2:
T1=8T2
Using the proportional relationship, we can write:
T1=kn31andT2=kn32
where k is a constant.
3. Substituting the Time Periods:
Substituting these expressions into the time period equation gives:
kn31=8(kn32)
Simplifying this, we get:
n31=8n32
4. Taking the Cube Root:
Taking the cube root of both sides, we find:
n1=2n2
5. Finding Possible Values:
Now we need to find integer values for n1 and n2 that satisfy this relationship. Since n1 must be twice n2, we can set n2=x and n1=2x.
The principal quantum numbers must be positive integers. Thus, we can choose:
- If n2=1, then n1=2×1=2
- If n2=2, then n1=2×2=4
- If n2=3, then n1=2×3=6
- And so on...
However, since the problem does not specify a maximum limit for n, we can conclude that the pairs (n1,n2) can be:
- (2,1)
- (4,2)
- (6,3)
- etc.
6. Conclusion:
The possible values of n1 and n2 that satisfy the condition given in the problem are:
- n1=4 and n2=2 (as one of the valid pairs).
The time period T of an electron in a hydrogen atom is related to the principal quantum number n. The time period can be expressed as:
T∝n3
This means that the time period is directly proportional to the cube of the principal quantum number.
According to the problem, the time period of the electron in the initial state n1 is eight times that in the final state n2:
T1=8T2
Using the proportional relationship, we can write:
T1=kn31andT2=kn32
where k is a constant.
Substituting these expressions into the time period equation gives:
kn31=8(kn32)
Simplifying this, we get:
n31=8n32
Taking the cube root of both sides, we find:
n1=2n2
Now we need to find integer values for n1 and n2 that satisfy this relationship. Since n1 must be twice n2, we can set n2=x and n1=2x.
- If n2=1, then n1=2×1=2
- If n2=2, then n1=2×2=4
- If n2=3, then n1=2×3=6
- And so on...
- (2,1)
- (4,2)
- (6,3)
- etc.
The possible values of n1 and n2 that satisfy the condition given in the problem are:
- n1=4 and n2=2 (as one of the valid pairs).
Topper's Solved these Questions
- ATOMSBOOK - DC PANDEY ENGLISHCHAPTER - ATOMSEXERCISE - ASSERTION AND REASON
10 Videos
- ATOMSBOOK - DC PANDEY ENGLISHCHAPTER - ATOMSEXERCISE - MATCH THE COLUMNS
4 Videos
- ATOMSBOOK - DC PANDEY ENGLISHCHAPTER - ATOMSEXERCISE - Check point 12.3
15 Videos
- ALTERNATING CURRENTBOOK - DC PANDEY ENGLISHCHAPTER - ALTERNATING CURRENTEXERCISE - JEE MAIN
62 Videos
- CAPACITORSBOOK - DC PANDEY ENGLISHCHAPTER - CAPACITORSEXERCISE - OBJECTIVE_TYPE
1 Video
Similar Questions
Assertion: Angular momentum of single electron in any orbit or hydrogen type atom is independent of the atomic number of the element.
Reason: In ground state angular momentum is minimum
View SolutionIn Column I physical quantities corresponding to hydrogen and hydrogen like atom are given. In column II, powers of principal quantum number n are givem on which thosee physical quantities depend. Match the two columns.
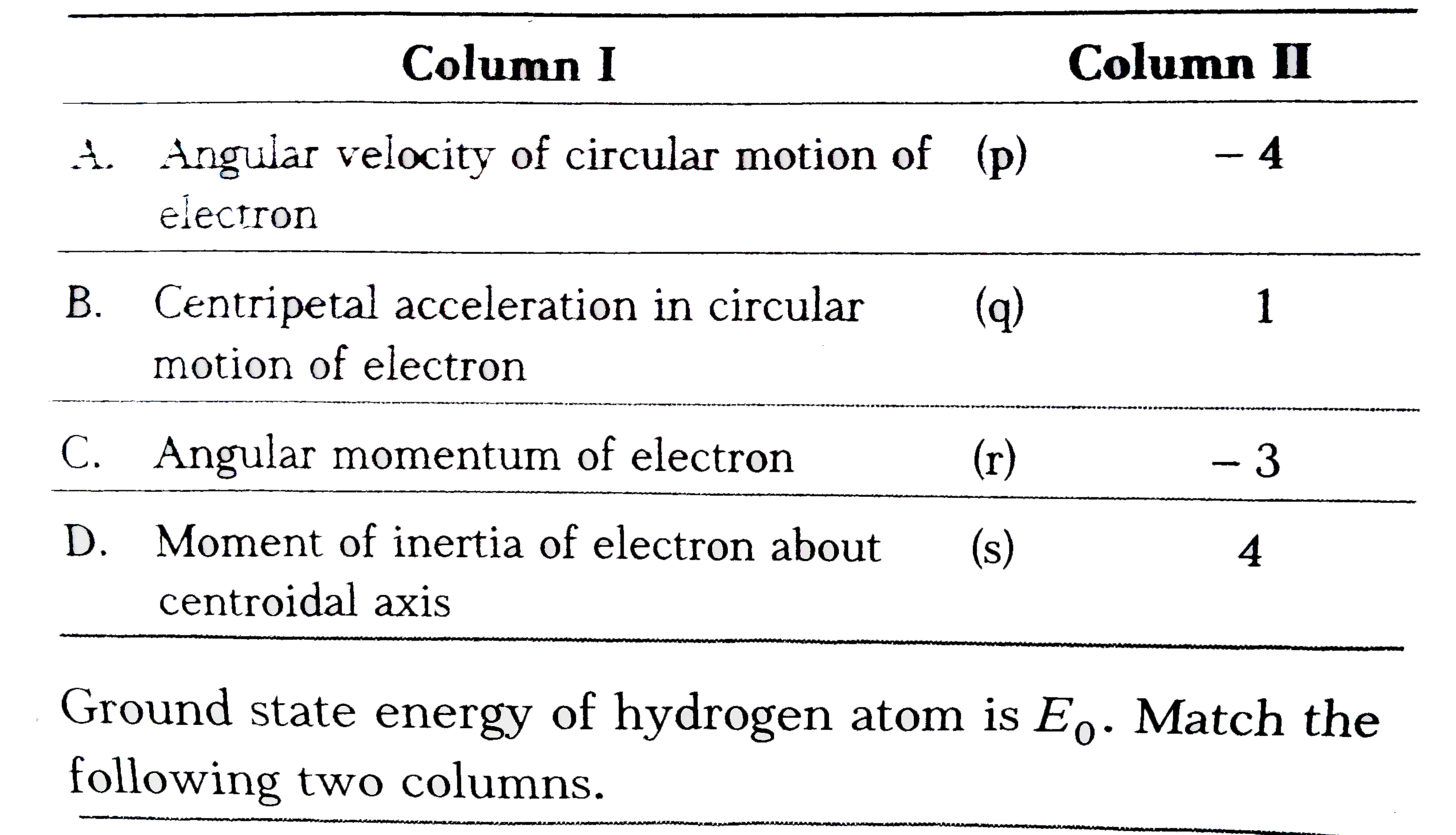
View SolutionX-rays were discovered by
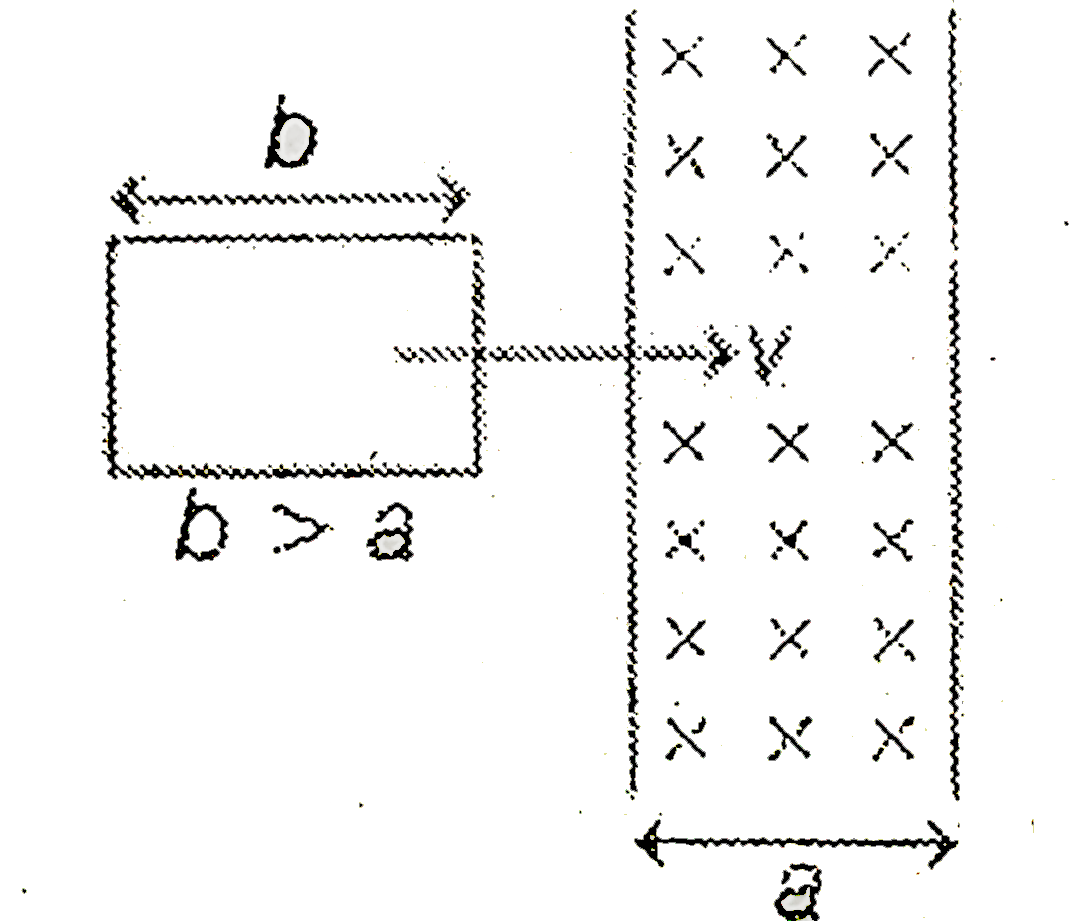
View SolutionIn the given arrangement, the loop is moved with consisant velocity in uniform magnetic field B in a restricted a region of width a. The time for which the emf is induced in the cirucit is
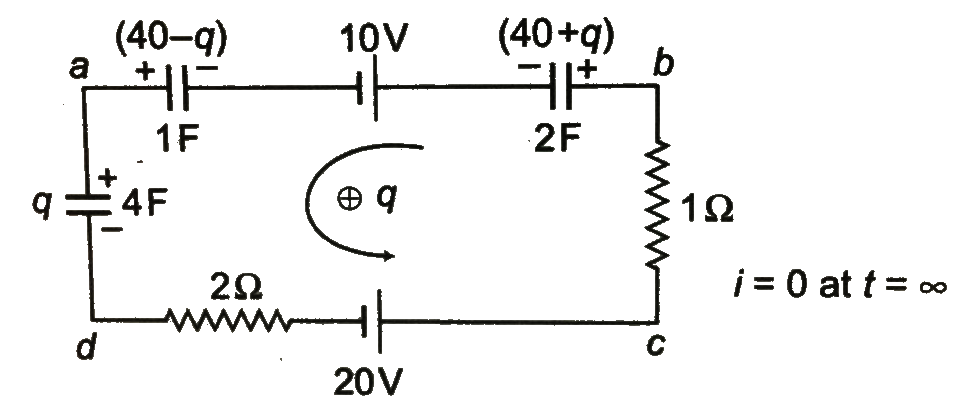
View SolutionIn the circuit shown as potential difference of 60V is appliled across AB. The potential difference between the points M and N is
View SolutionThe electron in a hydrogen atom makes a trapsition n_(1) to n_(2), where n_(1) and n_(2) are the principal quantum numbers of the two energy states. Assume Bohr's model to be velid. The time period of the electron in the initial state is eight time that in the final static. what are the possible value of n_(1) and n_(2) ?
View SolutionThe electron in a hydrogen atom makes a transition from n=n_(1) to n=n_(2) state. The time period of the electron in the initial state (n_(1)) is eigh times that in the final state (n_(2)) . The possible values of n_(1) and n_(2) are
View SolutionAn electron in a hydrogen atom makes a transition from n_(1) to n_(2) . If the time period of electron in the initial state is eight times that in the final state then Find the ratio n_(1)/n_(2)
View SolutionAn electron in a H2 atom makes a transition from n1→n2. The time period of electron in the initial state is eight times that in the final state. Then ratio of n1 to n2:
View SolutionThe electron in a hydrogen atom makes a transition from n = n_(1) to n = n_(2) state. The time period of the electron in the initial state (n_(1)) is eight times that in the final state (n_(2)) The possible values of n_(1) and n_(2) are
View SolutionAn electron in a hydrogen atom makes a transition nl→n2, where n1andn2 are principal quantum numbers of the states. Assume the Bohr's model to be valid. The time period of the electron in the initial state is eight times to that of final state. What is ratio of n2/n2
View SolutionAn electron in a hydrogen atom makes a transition nl→n2, where n1andn2 are principal quantum numbers of the states. Assume the Bohr's model to be valid. The time period of the electron in the initial state is eight times to that of final state. What is ratio of n2/n2
View SolutionAn electron in a hydrogen atom makes a transition nl→n2, where n1andn2 are principal quantum numbers of the states. Assume the Bohr's model to be valid. The time period of the electron in the initial state is eight times to that of final state. What is ratio of n2/n2
View SolutionThe electron in a hydrogen atom makes a transition from n = n_(1) to n = n_(2) state. The time period of the electron in the initial state is eight times that in the electron in the initial state is eight times that is the final state. The possible values of n_(1) and n_(2) are
View SolutionThe electron in a hydrogen atom makes a transition n1→n2, where n1 and n2 are the principle quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. the possible values of n1 and n2 are
View Solution

