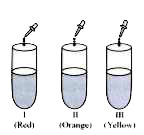
The given figure shows the colour changes in test tubes I, II and III, when methyl orange indicator is added to them. The respective solutions in test tubes I, II and III are
Carbonated drink, curd, spinach juice
Curd, window cleaner, glucose solution
Lime water, lemon juice, washing soda
Tamarind juice, glucose solution, baking soda solution
The correct Answer is:D
Tamarind juice contains tartaric acid, so gives red colour by adding methyl orange indicator. Glucose solution being neutral, does not change the colour of indicator. Whereas baking soda solution is a base, hence become yellow on addition of methyl orange indicator.
Topper's Solved these Questions
- NSO QUESTION PAPER 2019- 20 SET - BBOOK - SCIENCE OLYMPIAD FOUNDATION CHAPTER - NSO QUESTION PAPER 2019- 20 SET - BEXERCISE - ACHIEVERS SECTION
2 Videos
- NSO QUESTION PAPER 2018-19 SET ABOOK - SCIENCE OLYMPIAD FOUNDATION CHAPTER - NSO QUESTION PAPER 2018-19 SET AEXERCISE - LOGICAL REASONING
8 Videos
- NSO QUESTION PAPER 2019-20 SET ABOOK - SCIENCE OLYMPIAD FOUNDATION CHAPTER - NSO QUESTION PAPER 2019-20 SET AEXERCISE - SCIENCE
3 Videos
Similar Questions
Knowledge Check
A student appears for tests I, II and III. The student is successful if the passes either in tests I and II or tests I and III. The probabilities of the student passing in tests I, II and III are p,q and 12, respectively. If the probability that the student is successful, is 12, then
Ap=q=1B1Cp=1,q=0D3/4When dilute hydrochloric acid is added to zinc pieces taken in a test tube
Ano change takes placeBthe colour of the solution becomes yellowCa pungent smelling gas gets liberatedDsmall bubbles of hydrogen gas appear on the surface of zinc pieces.A student appears for tests I, II and III. The student is successful if the passes either in tests I and II or tests I and III. The probabilities of the student passing in tests I, II and III are p,q and 12, respectively. If the probability that the student is successful, is 12, then
Ap=q=1Bp=q=12Cp=1,q=0Dp=1, q=12
A student appears for tests I, II and III. The student is successful if he passes either in tests I and II or tests I and III. The probabilities of the student passing in tests I, II, and III are, respectively, p, q, and 1/2. then p(1+q)=
View SolutionBoojho took some grains of boiled rice in test tube 'A' and Paheli took boiled and chewed rice in test tube 'B'. Both of them poured 1-2 drops of iodine solution into the test tube and observed the colour change. What colour change would they have observed? Give reasons for your answer.
View SolutionA student appears for tests I, II and III. The student is successful if the passes either in tests I and II or tests I and III. The probabilities of the student passing in tests I, II and III are p,q and 12, respectively. If the probability that the student is successful, is 12, then
View SolutionA student appears for tests I. II and III. The student is considered successful if he passes in test I. II or I. III or all the three. The probabilities of the student passing in test I. II and III are m. n and 12 respectively. If the probability of the student to be successful is 12 then which one of the following is correct ?
View SolutionSheela designs an experiment to test the effect of solute concentration during osmosis. She takes four equal sized potato cubes having an identical weight and places each cube in four separate test tubes I, II, III, and IV, containing 1%, 2%, 3%, and 4% sucrose concentrations respectively. After one hour, she removes the cubes from the test tubes, blots the pieces with a paper towel, and weighs them to find the final mass of each cube. The solution in test tube Ill was hypertonic to the cytoplasm of the potato cells. What change will be observed in the potato cube of test tube Ill?
View Solution

