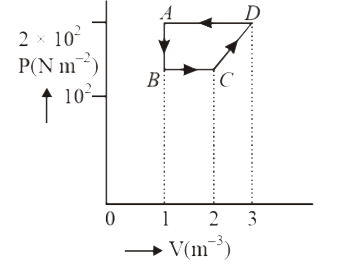
The P-V diagram of a gas undergoing a cyclic process (ABCDA) is shown in the graph, where P is in units of Nm−2 and V in cm3. Identify the incorrect statement.
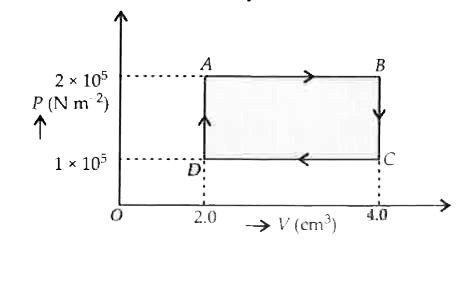
0.4 J of work is done by the gas from A to B
0.2 J of work is done on the gas from C to D.
No work is done by the gas from B to C.
Work is done by the gas from B to C and on the gas from D to A.
The correct Answer is:D
In going from B to C and from D to A, V = constant.
So, ΔV=0.∴W=PΔV=0
Topper's Solved these Questions
- THERMODYNAMICSBook:MTG GUIDEChapter:THERMODYNAMICSExercise:NEET (ITALS)
Explore 25 Videos
- THERMODYNAMICSBook:MTG GUIDEChapter:THERMODYNAMICSExercise:AIPMT/NEET (MCQs)
Explore 26 Videos
- THERMODYNAMICSBook:MTG GUIDEChapter:THERMODYNAMICSExercise:AIPMT/NEET (MCQs)
Explore 26 Videos
- PROPERTIES OF BULK MATTERBook:MTG GUIDEChapter:PROPERTIES OF BULK MATTERExercise:AIPMT/NEET (MCQs)
Explore 40 Videos
- WORK, ENERGY AND POWERBook:MTG GUIDEChapter:WORK, ENERGY AND POWERExercise:AIPMT/NEET (MCQs)
Explore 34 Videos
Similar Questions
"Two systems in thermal equilibirum with a third system separately are in thermal equilibrium with each other". The aove statement is
View SolutionIf ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true ?
View SolutionIf ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true ?
View SolutionThe total radiant energy per unit area, normal to the direction of incidence, received at a distance R from the centre of a star of radius r whose outer surface radiates as a black body at a temperature TK is given by
(where σ is Stefan's constant)
View SolutionA ball moving with velocity 2 m/s collides head on with another stationary ball of double the mass. If the coefficient of restitution is 0.5, then their velocities (in m/s) after collision will be
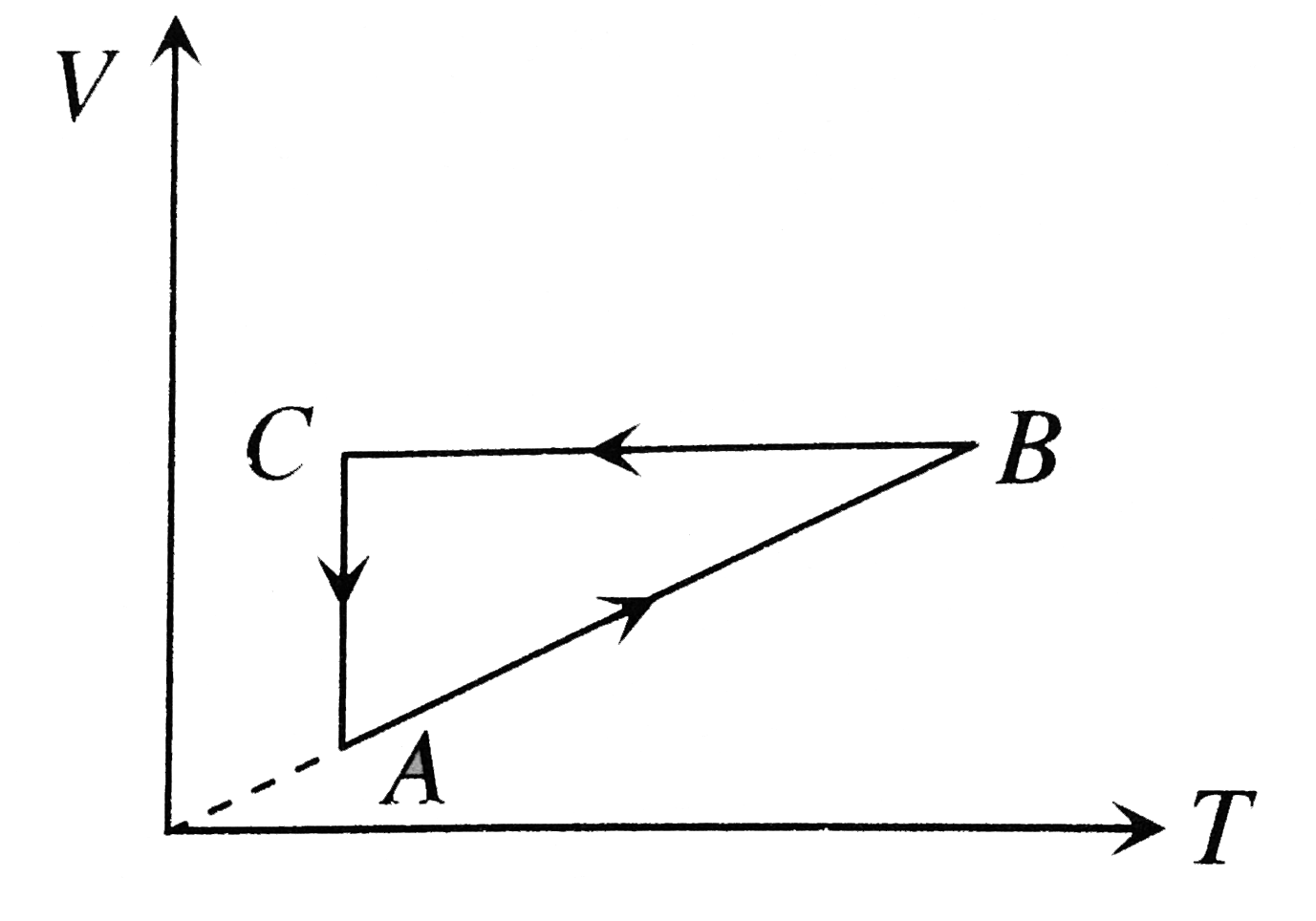
View SolutionA cyclic process ABCA is shown in the V−T diagram process on the P−V
View Solutionp-T curve for a cyclic process is as shown P-V graph for this process will be:
View SolutionAn ideal gas underoges cyclic process of ABCDA as shown in Given P−V diagram (figure)
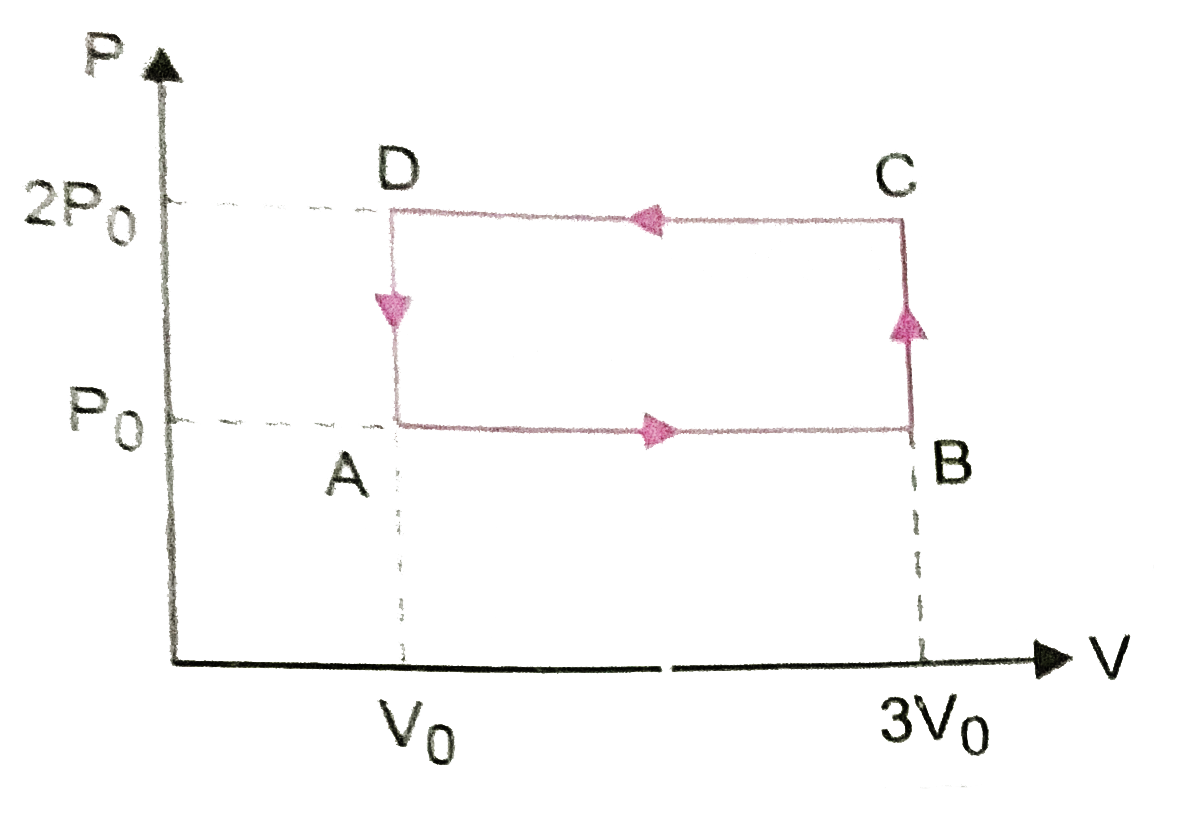
The amount of work done by the gas is
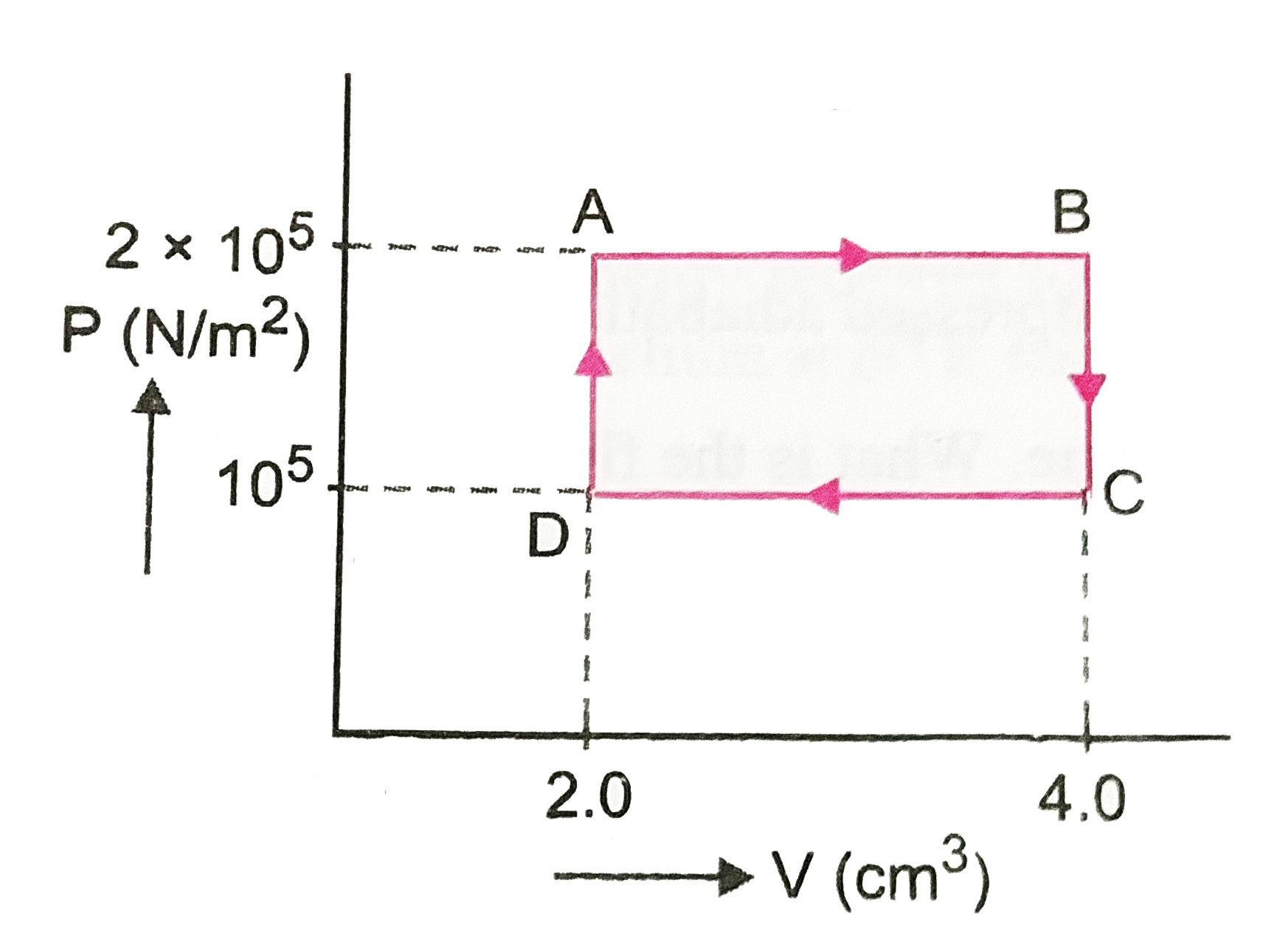
View SolutionThe P−V diagram of a gas undergoing a cyclic process ABCDA is shown in (figure). Where P is in N/m2 and V is in cm3. Identify the incorrect statement
View SolutionThe P-V diagram of a gas system undergoing cyclic process is shown here. The work done during isobaric compression is
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in givend p−V diagram. The amount of work done by the gas is
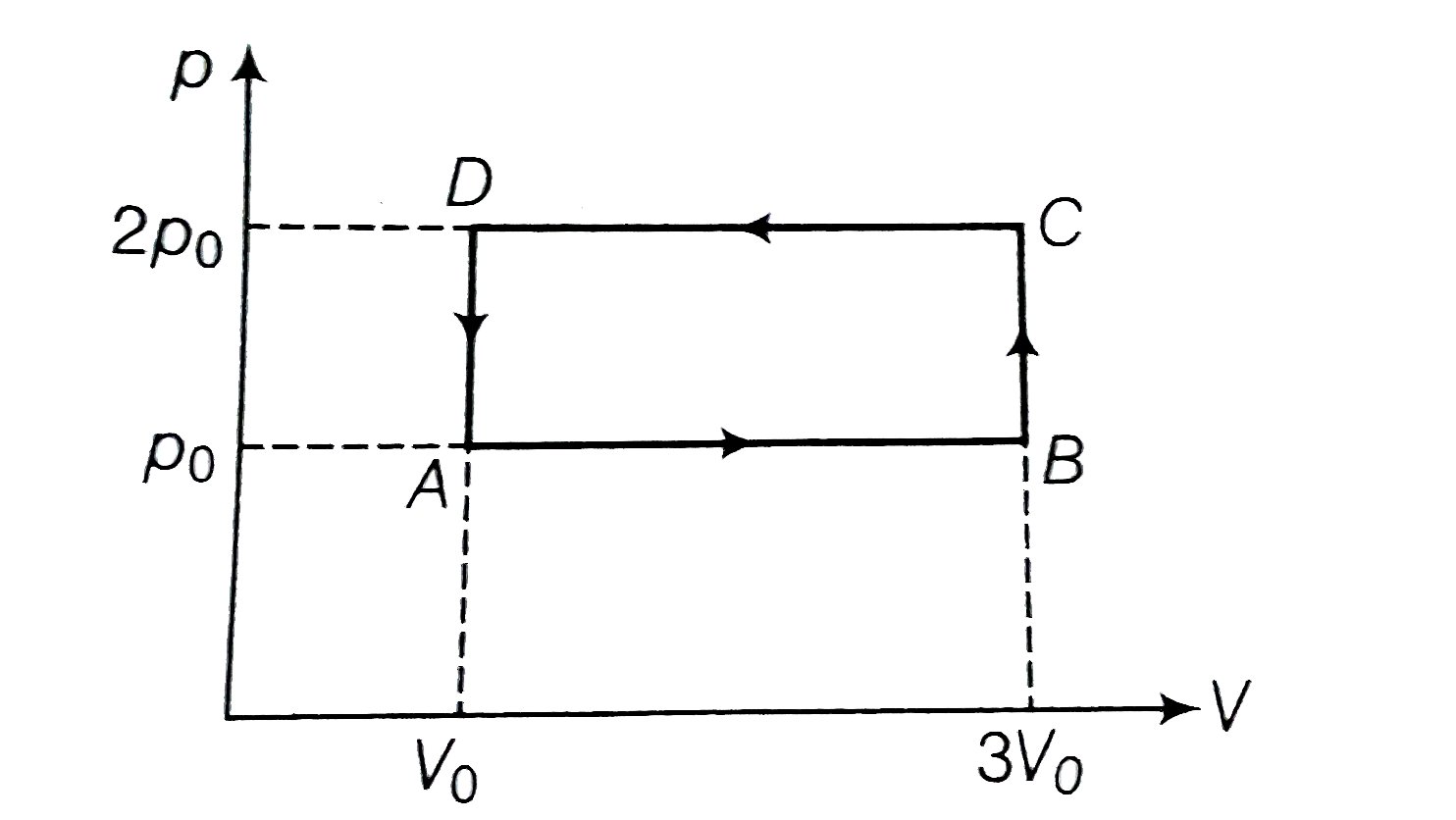
View SolutionOne mole of an ideal gas undergoes a cyclic process ABCDA as shown in the P-V diagram, The net work done in the process is (1 atm=10^(6) dyne cm^(-2)) ltbvrgt
View SolutionThe P-V diagram of a gas undergoing a cyclic process ABCDA is shown in graph where P is in N//m^2 and V is in (Cm)^3 . Identify the incorrect statement :
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in given P - V diagram. The amount of work done by the gas is ___
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in givend p-V diagram. The amount of work done by the gas is
View Solution

