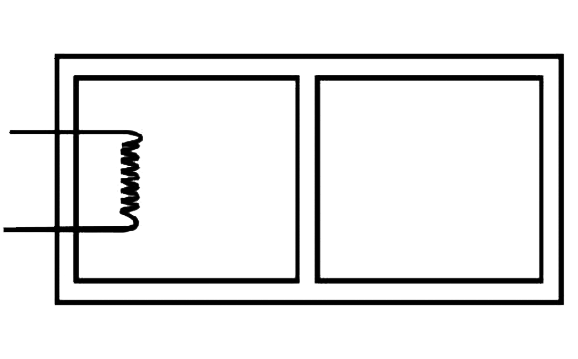
The rectangular box shown in Fig has partition which can slide without friction along the length of the box. Initially each of the two chambers of the box has one mole of a mono-atomic ideal gas (λ=5/3) at a pressure P0, volume V0 and temperature T0. The chamber on the left is slowly heated by an electric heater. The walls of the box and the lead wires of the heater is negligible. The gas in the left chamber expands pushing the partition until the final pressure in both chambers becomes 243P0/32. Determine (i) the final temperature of the gas in each chamber and (ii) the work done by the gas in the right chamber.
Step by step video & image solution for The rectangular box shown in Fig has partition which can slide without friction along the length of the box. Initially each of the two chambers of the box has one mole of a mono-atomic ideal gas (lambda=5//3) at a pressure P_0, volume V_0 and temperature T_0. The chamber on the left is slowly heated by an electric heater. The walls of the box and the lead wires of the heater is negligible. The gas in the left chamber expands pushing the partition until the final pressure in both chambers becomes 243P_0//32. Determine (i) the final temperature of the gas in each chamber and (ii) the work done by the gas in the right chamber. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBOOK - SL ARORACHAPTER - THERMODYNAMICSEXERCISE - Problems for self practice
97 Videos
- THERMODYNAMICSBOOK - SL ARORACHAPTER - THERMODYNAMICSEXERCISE - Exercise
342 Videos
- THERMODYNAMICSBOOK - SL ARORACHAPTER - THERMODYNAMICSEXERCISE - Exercise
342 Videos
- Thermal Properties of MatterBOOK - SL ARORACHAPTER - Thermal Properties of MatterEXERCISE - Exercise
449 Videos
- Units and MeasurementsBOOK - SL ARORACHAPTER - Units and MeasurementsEXERCISE - Exercise
499 Videos
Similar Questions
A rectangualr box (shown in figure) has a movable and smooth portition which can slide along the length of the box. Both chambers contains 1 mole of monoatomic gas (γ=53) at a pressure walls of box and partition are thermally insulated. Due to heating, gas in left chamber expands until pressure in both chambers become 32P0 determine
(a) The final temperature of gas in each chamber
(b) The work done by the gas in the right chamber.
View SolutionA rectangular box has a volume 48 and the sum of the length of the twelves edges of the box is 48. The largest integer that can be the length of the box is
View Solution
Knowledge Check
A sample of gas in a box is at pressure P0 and temperature T0. If number of molecules is doubled and total kinetic energy of the gas is kept constant, then final temperature and pressure will be
AT0.P0BT0,2P0CT02,2P0DT02,P0An ideal gas is at pressure P and temerature T in a box, which is kept in vaccum with in a large container. The wall of the box is punctured. What happens as the gas occupies entire container?
AIt's temperature fallsBIts temperature risesCIts temperature remains the sameDUnpredicatableOne mole of a monoatomic ideal gas occupies two champers of a cylinder partioned by means of a movable pision. The walls of the cylinder as well as the piston are thermal insulators. Initially equal amounts of gas fill both the chambers at (P0,V0,T0). A coil is burnt in the left chamber which absorbs heat and expands , pushing the piston to the right . The gas on the right chamber is compressed until to pressure becomes 32P0.
_S01_111_O01.png)
The work done on the gas in the right chamber isA92P0V0B−92P0V0C132P0V0D172P0V0
A thermally insulating cylinder has a thermally insulating and frictionless movable partition in the middle, as shown in the figure below. On each side of the partition, there is one mole of an ideal gas , with specific heat at constant volume, Cv=2R. Here, R is the gas constant. Initially, each side has a volume V0 and temperature T0. The left side has an electric heater, which is turned on at very low power to transfer heat Q to the gas on the left side. As a result the partition moves slowly towards the right reducing the right side volume to V02 . Consequently, the gas temperature on the left and the right sides become TL and TR, respectively. Ignore the changes in the temperatures of the cylinder, heater and the partition.
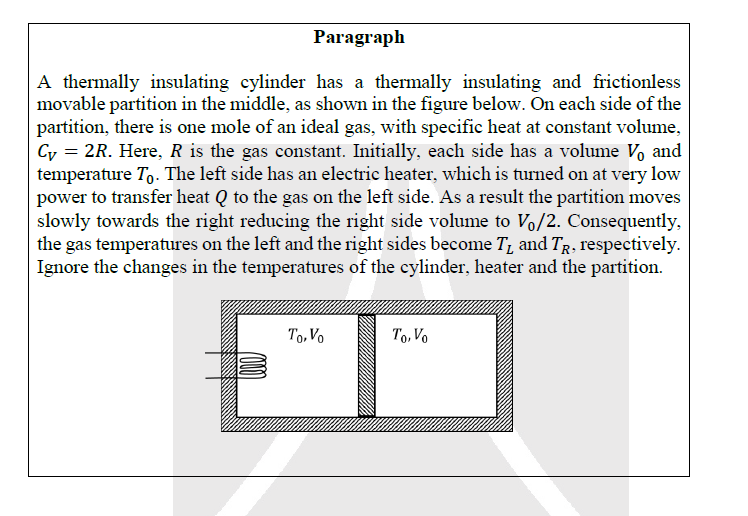
Value of TRT0
View SolutionAn exhausted chamber with nonconducting walls is connected through a valve to the atmosphere where the pressure is p0and the temperature is T0.The valve is openeds lightly and air flows into the chamber until the pressure with in the chamber is p0.Assuming the air to be have like an ideal gas with constant heat capacities, the final temperature of the air in the chamber is
View SolutionOne mole of O2 gas is contained in a box of volume V=2m3 at pressure P0 and temperature 300K The gas is now heated to 600 K and the molecules now get dissociated into oxygen atoms. The new pressure of the gas is
View SolutionConsider a black body radiation in a cubical box at absolute temperature T. If the lengh of each side of the box is doubled and the temperature of the walls of the box and that of the radiation is halved then the total energy
View SolutionA mixture of ideal gas containing 5 moles of monatomic gas and 1 mole of rigid diatomic gas is initially at pressure P0 volume V0 and temperature T0. If the gas mixture is adiabatically compressed to a volume V0/4, then the correct statement(s) is(are) : (Given R is gas constant)
View Solution

