The speed of sound in hydrogen gas at certain temperature is v ms Find the speed of sound in a gaseous mixture containing 2 moles of oxygen and 1 mole of hydrogen gas, at the same temperature. Assume the gases do no react at the ordinary temperature.
For a mixture of non reacting gases,
n1γ1−1+n2γ2−1=n1+n2γmix−1
For given problem
n1=2,n2=2,γ1=75,γ2=75,γmix=1.4.
Mmix=n1m1+n2m2n1+n2
Here, m1=32,m2=2,Mmix=22.
Vmix=√γmixRTMmix and VH=√γH2RTMH2
gammaVmix=√γmixMmix×MHγH×VH=V√11ms
Topper's Solved these Questions
- SOUND WAVES AND DOPPLER EFFECTBOOK - CENGAGE PHYSICSCHAPTER - SOUND WAVES AND DOPPLER EFFECTEXERCISE - Single Correct
252 Videos
- SOUND WAVES AND DOPPLER EFFECTBOOK - CENGAGE PHYSICSCHAPTER - SOUND WAVES AND DOPPLER EFFECTEXERCISE - Multiple Correct
44 Videos
- SOUND WAVES AND DOPPLER EFFECTBOOK - CENGAGE PHYSICSCHAPTER - SOUND WAVES AND DOPPLER EFFECTEXERCISE - Exercise 6.2
72 Videos
- RIGID BODY DYNAMICS 2BOOK - CENGAGE PHYSICSCHAPTER - RIGID BODY DYNAMICS 2EXERCISE - Interger
2 Videos
- SUPERPOSITION AND STANDING WAVESBOOK - CENGAGE PHYSICSCHAPTER - SUPERPOSITION AND STANDING WAVESEXERCISE - Comprehension Type
5 Videos
Similar Questions
A man is watching two trains, one leaving and the other coming in with equal speed of 4m/s. If they sound their whistles, each of frequency 240 Hz, the number of beats heard by the man (velocity of sound in air is 320m/s will be equal to
View SolutionWhich of the following statements are incorrect?
View SolutionA person riding on a merry go round emits a sound wave of a certain frequency Does a person at the centre observe the Doppler effect?
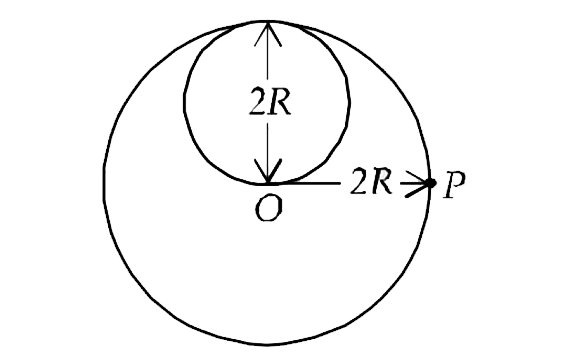
View SolutionA lamina is made by removing a small disc of diameter 2R from a bigger disc of uniform mass density and radius 2R, as shown in the figure. The moment of inertia of this lamina about axes passing though O and P is IOandIP respectively. Both these axes are perpendicular to the plane of the lamina. The ratio IPIO ot the nearest integer is
View SolutionA string of length L, fixed at its both ends is vibrating in its 1st overtone mode. Consider two elements of the string of the same small length at positions l1=0.2Landl2=0.45L from one end.
If K1andK2 are their respective maximum kinetic energies,then
View SolutionThe speed of sound in oxygen (O2) at a certain temperature is 460ms−1. The speed of sound in helium (He) at the same temperature will be (assume both gases to be ideal)
View SolutionThe speed of sound in hydrogen gas at certain temperature is v ms Find the speed of sound in a gaseous mixture containing 2 moles of oxygen and 1 mole of hydrogen gas, at the same temperature. Assume the gases do no react at the ordinary temperature.
View SolutionAt the room temperature the velocity of sound in O2 gas is v. Then in mixture of H2 and O2 gas the speed of sound at same temperature :-
View Solution(a) Calculate the speed of sound in hydrogen gas at 300 K
(b) At what temperature the speed in oxygen will be same as above. [Assume oxygen molecules to remain diatomic]
View SolutionLet speed of sound waves in hydrogen gas at room temperature is v0. What will be the speed of sound waves in a room which contains an equimolar mixture of hydrogen and 'He' at same temperature :-
View Solutionसमान्य ताप एवं दाब पर हाइड्रोजन गैस में ध्वनि की चाल और ऑक्सीजन में ध्वनि की चाल का अनुपात क्या होगा ?
View Solutionकिसी निश्चित ताप पर ऑक्सीजन (O2) में ध्वनि की चाल 460 मी/से है। इसी ताप पर हीलियम (He) में ध्वनि की चाल (दोनों गैसों को आदर्श गैस मानते हुए) होगी :
View Solutionএকটি নির্দিষ্ট তাপমাত্রায় অক্সিজেন গ্যাসে শব্দের বেগ 460 m/s | ওই একই তাপমাত্রায় হিলিয়াম গ্যাসে শব্দের বেগ (ধরা হয়েছে দুটি গ্যাসই আদর্শ)
View SolutionThe speed of sound in oxygen (O_(2)) at a certain temperature 66" ms"^(-1) , The speed of sound in helium (He) at the same temperature will be assume both gases to be ideal)
View SolutionThe speed of sound in oxygen gas at room temperature is V. Speed of sound in the mixture of hydrogen and oxygen at the same temperature:
View SolutionThe speed of sound in oxygen (O2) at a certain temperature is 460ms−1. The speed of sound in helium (He) at the same temperature will be (assume both gases to be ideal)
View Solution

