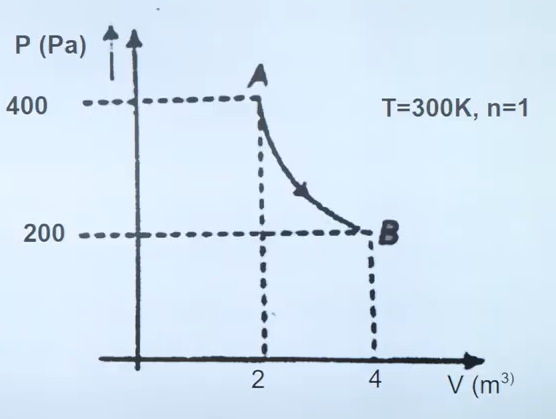
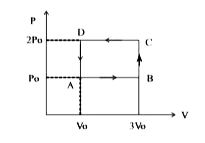
There are two process ABC and DEF. In which of the process is the amount of work done by the gas is greater ?
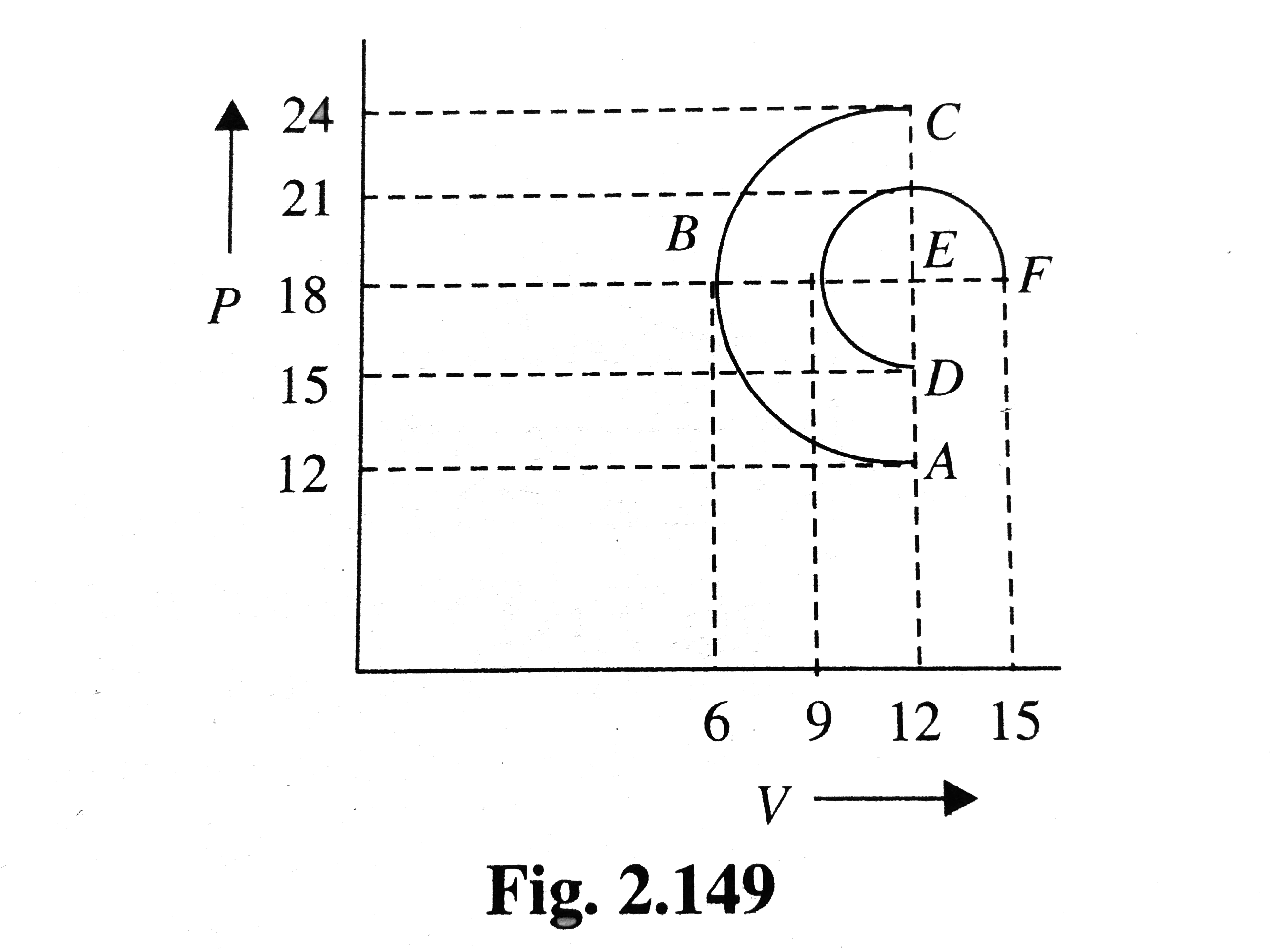
ABC
DEF
Equal in both process
it cannot be predicted
The correct Answer is:B
b. WABC=πr22=π(6)22=18π
WDEF=π×322+π×324+(15−12)×18
=6.75π+54
WDEF>WABC
Similar Questions
Knowledge Check
Find the work done by the gas in the process ABC.
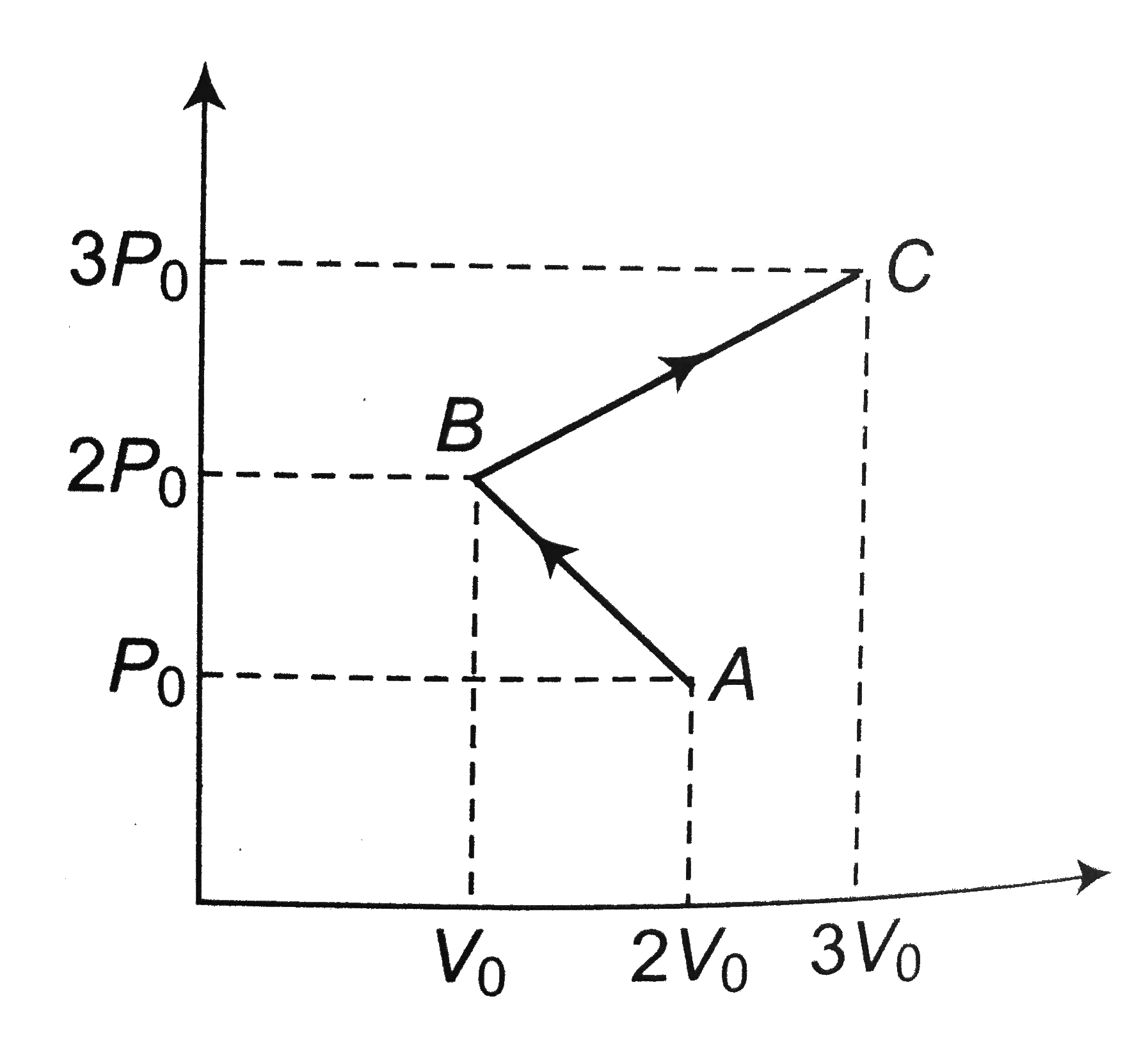 A32P0V0B52P0V0C72P0V0D4P0V0
A32P0V0B52P0V0C72P0V0D4P0V0The thermodynamic process in which no work is done on or by the gas is
AIsothermal processBIsochoric processCAdiabatic processDIsobaric processThe thermodynamic process in which no work is done on or by the gas is
Aisothermal processBadiabatic processCisochoric processDisobaric process
An ideal monatomic gas expands to twice its volume. If the process is isothermal, the magnitude of work done by the gas is W1. If the process is adiabatic, the magnitude of work done by the gas is Wa . Which of the following is true ?
View SolutionAn ideal gas is compressed to half its initial volume by means of several peocesses. Which of the process results in the maximum work done on the gas ?
View SolutionAn ideal gas is compressed to half its initial volume by means of several peocesses. Which of the process results in the maximum work done on the gas ?
View SolutionIn the cyclic process shown in the figure, the work done by the gas in one cycle is
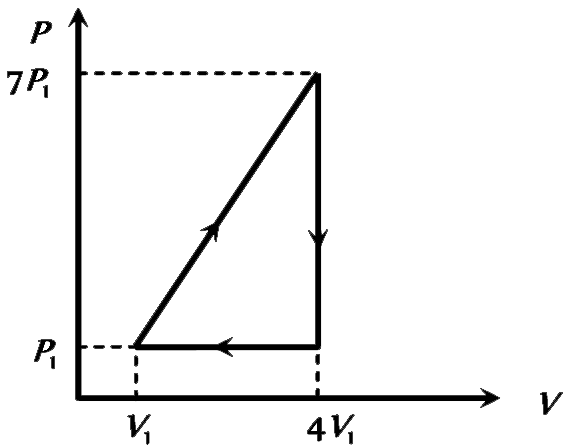
View SolutionStatement 1: Heat supplied to a gas is process is 100 J and work done by the gas in the same process is 120 J, then pressure of he gas in the process should increase.
because
Statement2: Work done by the gas is greater than the heat supplied to the gas. Hence, internal energy of the gas should decrease.
View Solution