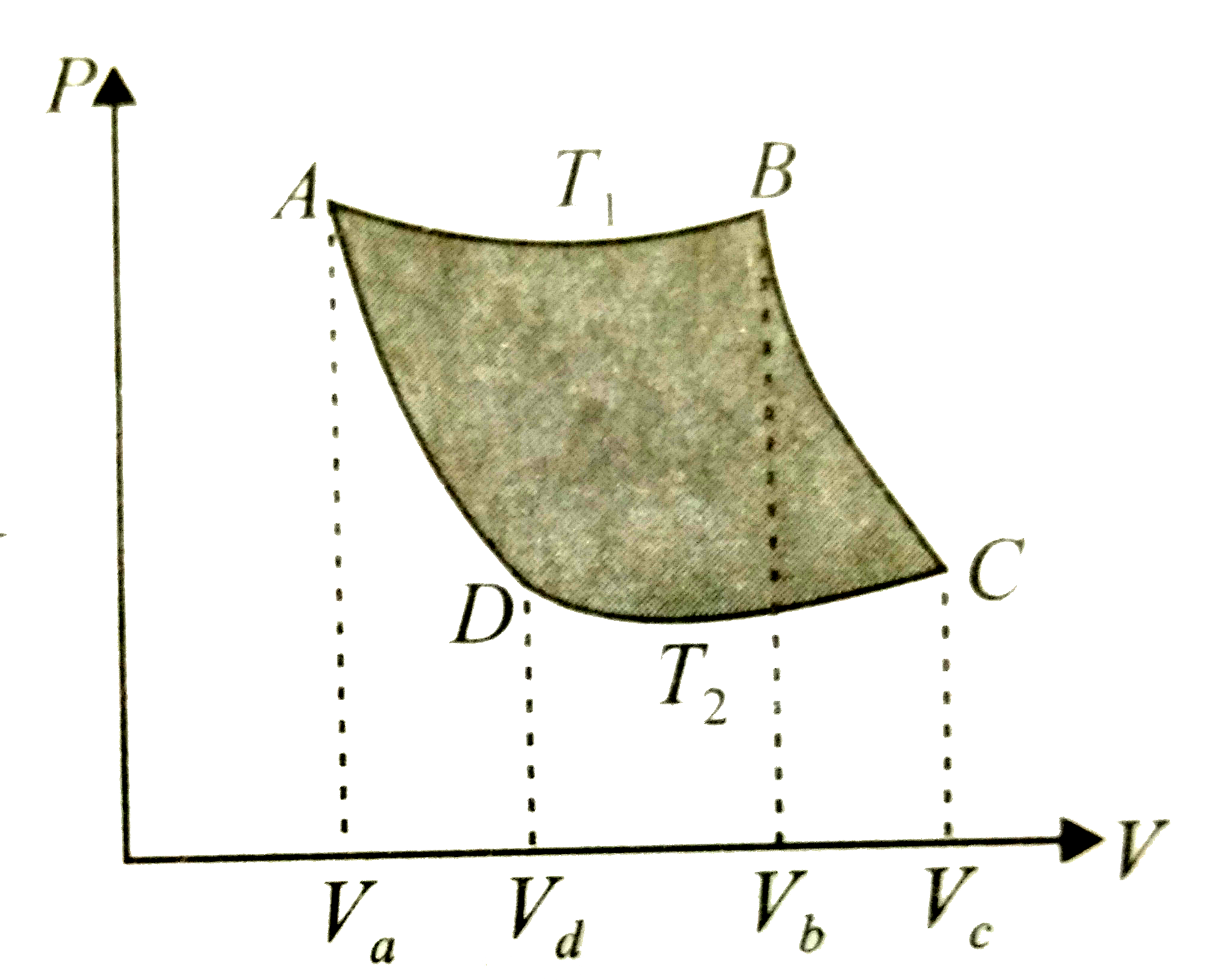
Two different adiabatic curves for the same gas intersect two isothermals at T1,andT2 as shown in P−V diagram, (figure). How does the ratio (Va/Vd) compare with the ratio (Vb/Vc) ?
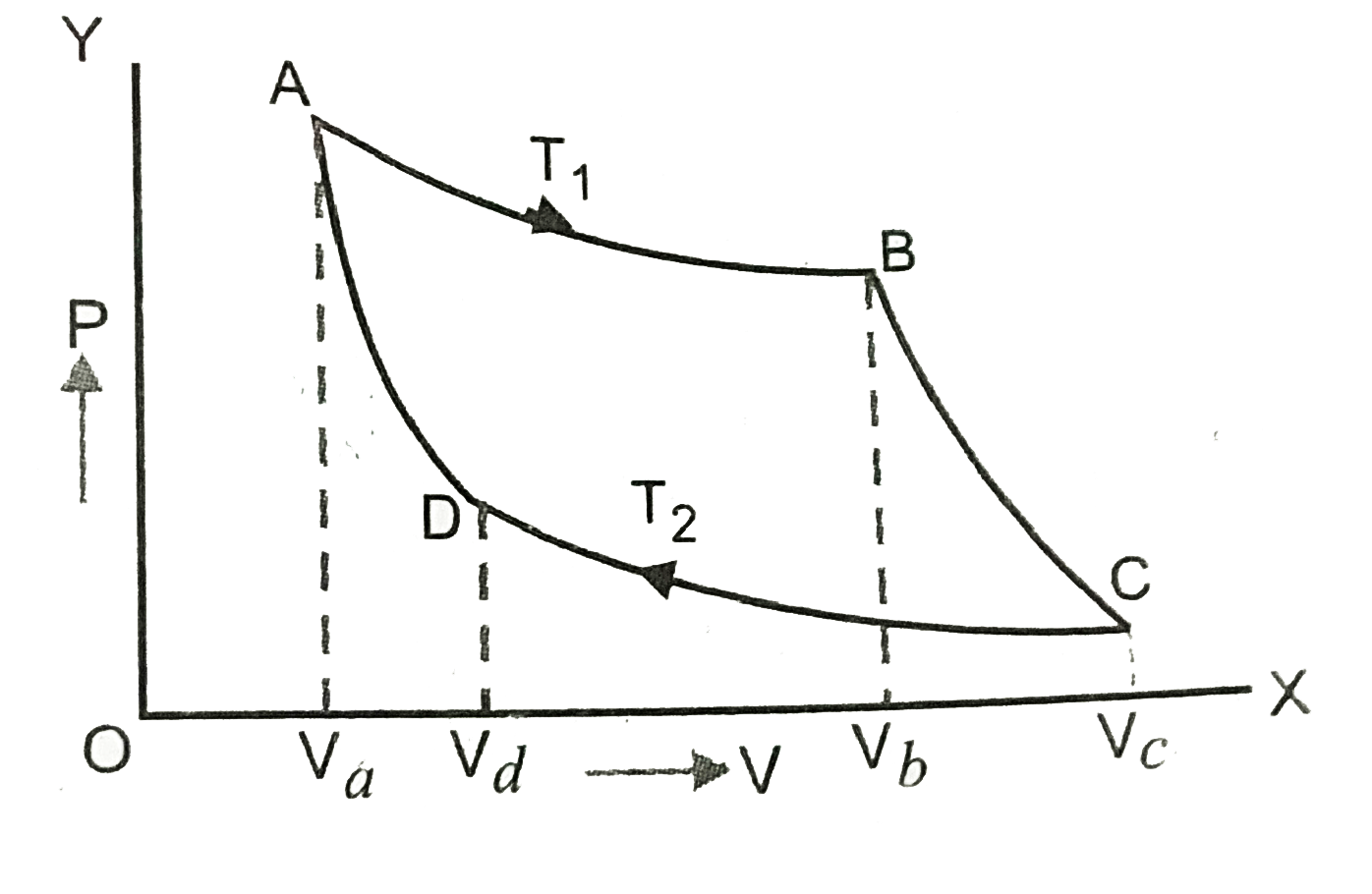
Step by step video & image solution for Two different adiabatic curves for the same gas intersect two isothermals at T_(1), and T_(2) as shown in P-V diagram, (figure). How does the ratio (V_(a)//V_(d)) compare with the ratio (V_(b)//V_(c)) ? by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBOOK - SL ARORACHAPTER - THERMODYNAMICSEXERCISE - Problems from competitive examinations
22 Videos
- THERMODYNAMICSBOOK - SL ARORACHAPTER - THERMODYNAMICSEXERCISE - Problems for self practice
97 Videos
- Thermal Properties of MatterBOOK - SL ARORACHAPTER - Thermal Properties of MatterEXERCISE - Exercise
449 Videos
- Units and MeasurementsBOOK - SL ARORACHAPTER - Units and MeasurementsEXERCISE - Exercise
499 Videos
Similar Questions
An ideal gas is taken through a cyclic thermodynamic process through four steps. The amounts of heat involved in these steps are Q1=5960J,Q2=−5585J,Q3=−2980JandQ4=3645J, respectively. The corresponding quantities of work involved are W1=2200J,W2=−825J,W3=−1100JandW4 respectively.
(1) Find the value of W4.
(2) What is the efficiency of the cycle
View SolutionAn ideal monoatomic gas is taken around the cycle ABCDA, wher co-ordinates of A, B, C and D on and D on P−V diagram are A(p,V),B(2p,V),C(2p,2V)andD(p,2V). Calculate work done during the cycle.
View SolutionA faulty thermometer reads 5∘C melting ice and 99∘C in steam. Find the correct temperature in .∘F when this faulty thermometer reads 52∘C.
View SolutionCalculate the number of astronomical units in one metre.
View SolutionA monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate.
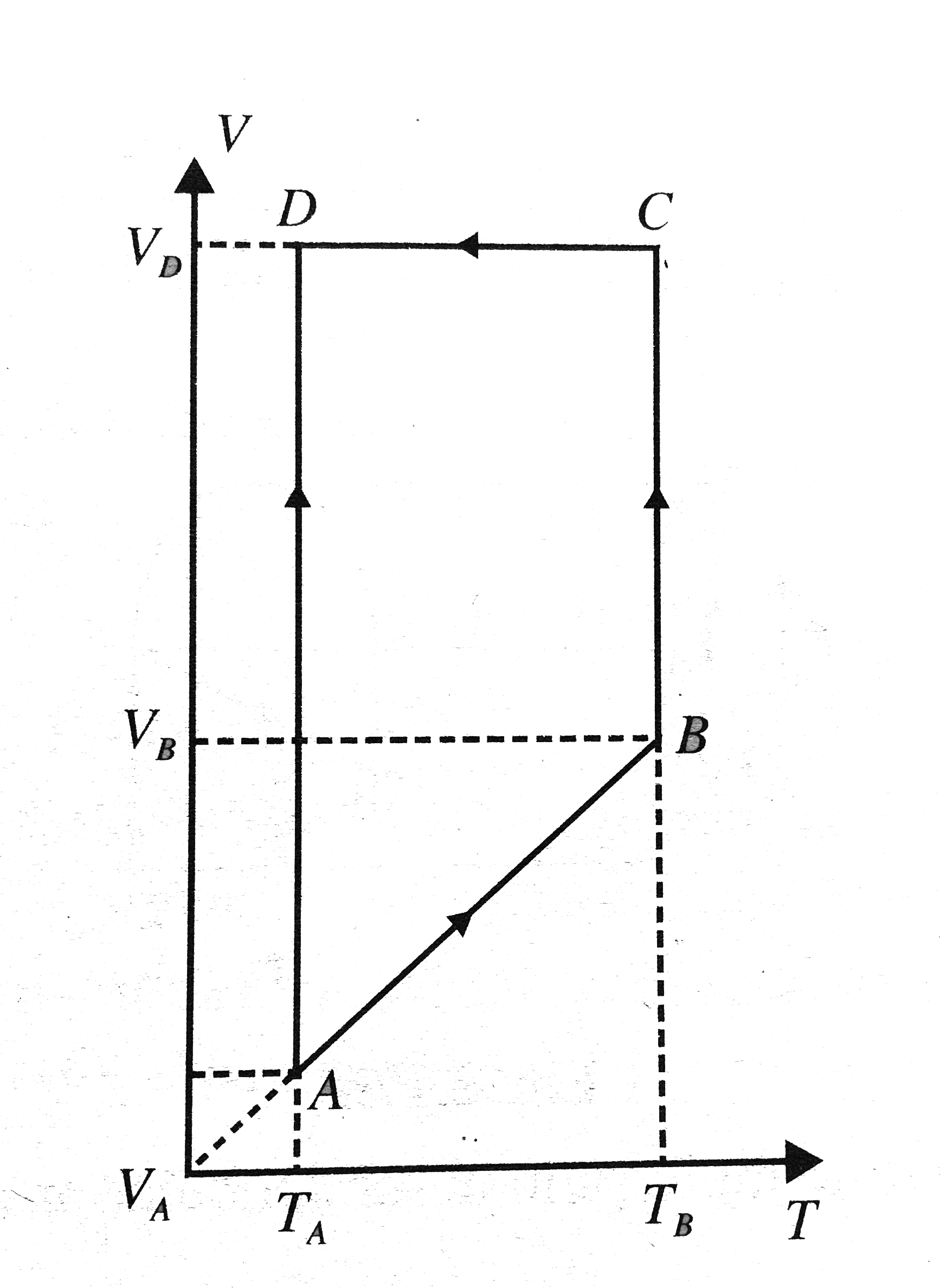 Heat absorbed or released by the gas in process B→C
Heat absorbed or released by the gas in process B→C
View SolutionA monatomic idea gas of 2 mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are V_(B)//V_(A)=2 and V_(D)//V_(A)=4 . If the temperature T_(A) at A is 27^(@)C , and gas constant is R . Calculate. Heat absorbed or released by the gas in process C rarr D .
View SolutionA monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate.
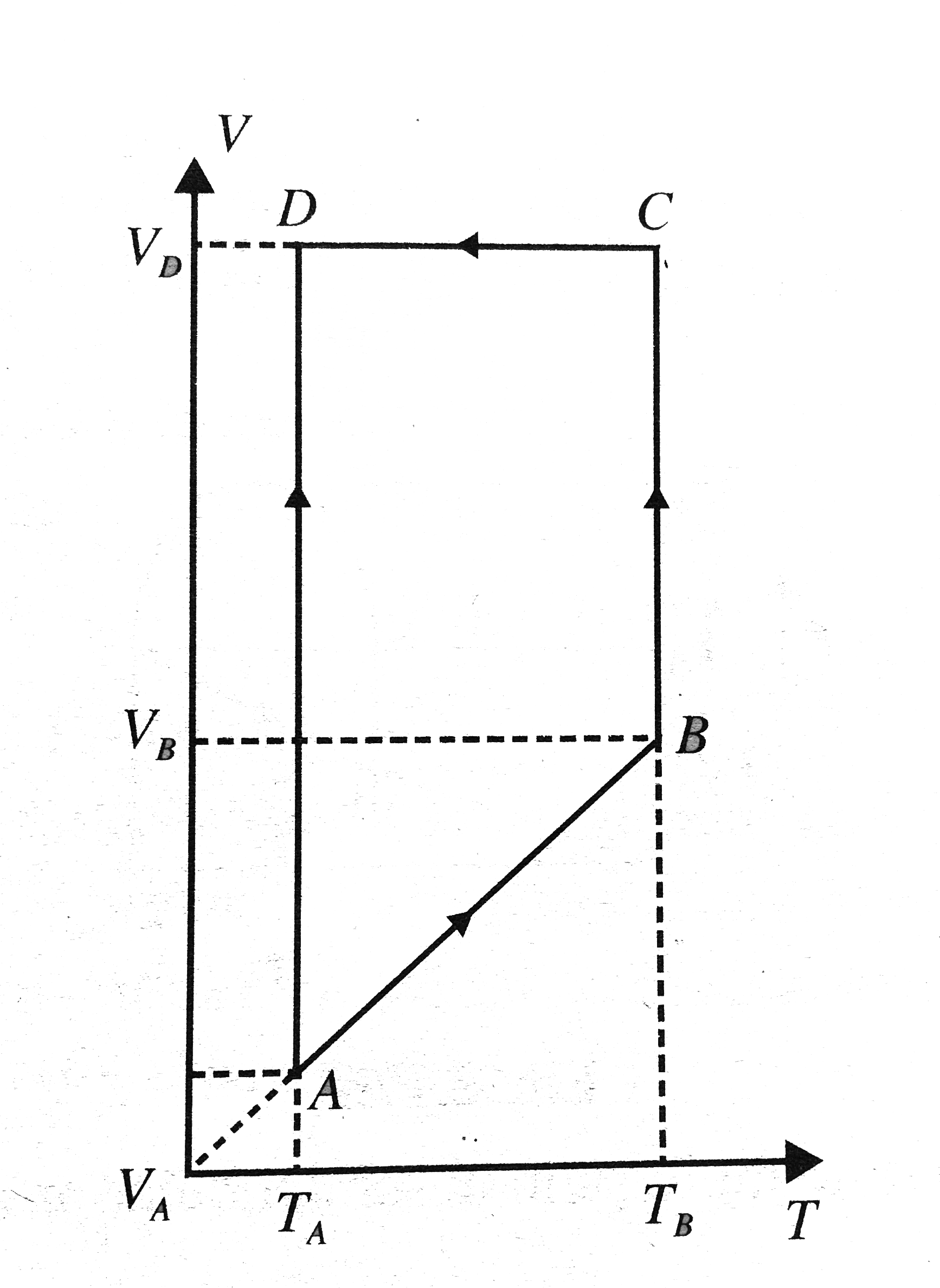
Heat absorbed or released by the gas in procedd D→A.
View SolutionIn the following P-V diagram two adiabatics cut two isothermals at temperature T_(1) and T_(2) (fig). The value of V_(a)/V_(d) will be
View SolutionTwo different adiabatic curves for the same gas intersect two isothermals at T1,andT2 as shown in P−V diagram, (figure). How does the ratio (Va/Vd) compare with the ratio (Vb/Vc) ?

View Solution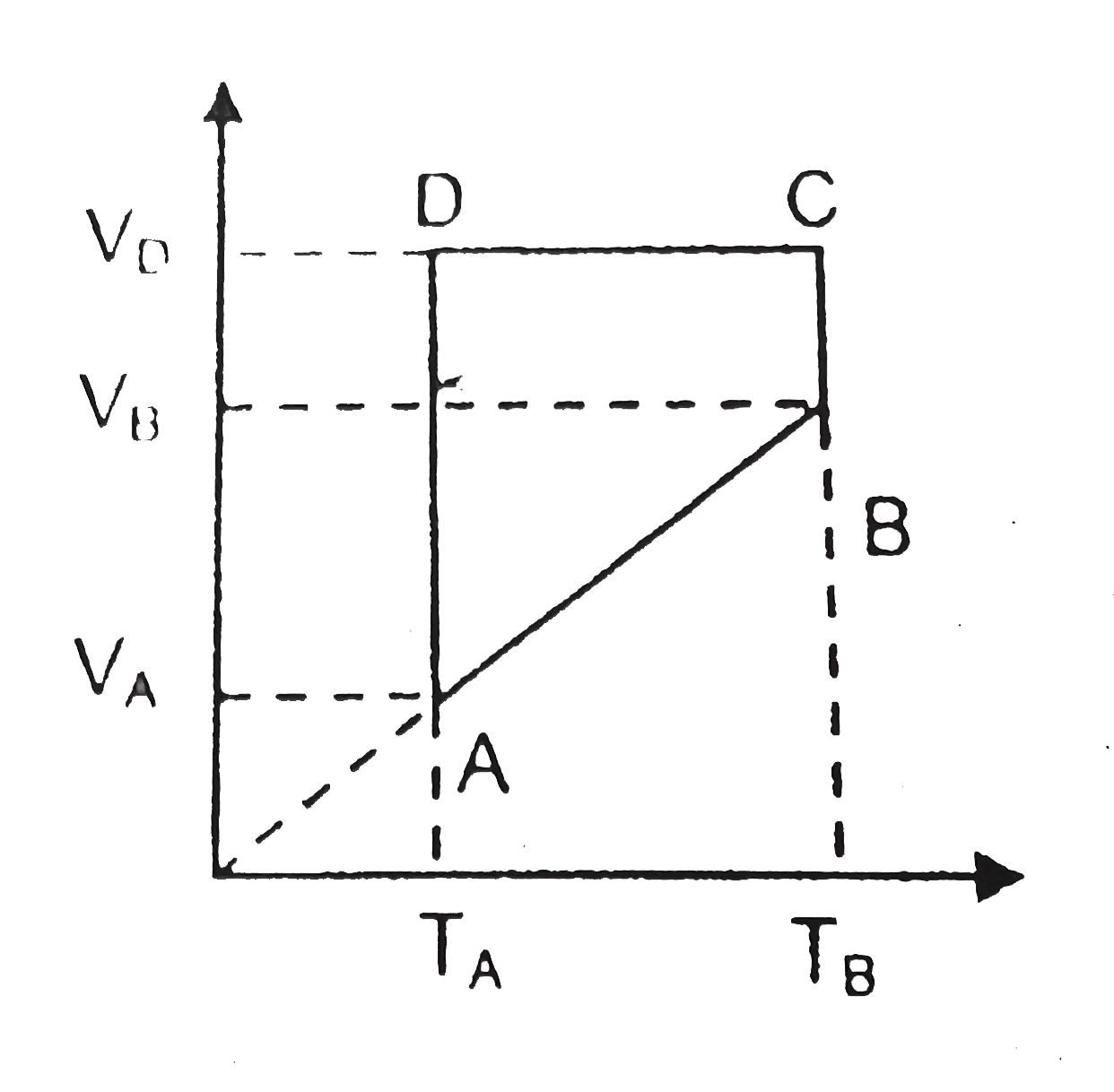
A monoatomic ideal gas of two moles is taken through a cyclic process starting from A as shown
VBVA=2 and VDVA=4
Temperature TA at A is 27∘C.
Q. Temperature at C is
View SolutionTwo different adiabatic parts for the same gas intersect two isothermals at T1andT2 shown in P-V diagram . Then the ratio of VaVb will be
View SolutionTwo cars of same mass are moving with velocities v_(1) and v_(2) respectively. If they are stopped by supplying same breaking power in time t_(1) and t_(2) respectively then (v_(1))/(v_(2)) is
View Solutionदो ताप T1 व T2 पर रुद्वोष्म वक्र चित्रानुसार है, Vb/Vc. का मान होगा।
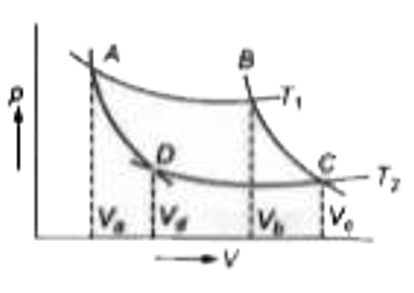
View SolutionTwo different adiabatic parts for the same gas intersect two isothermals at T1andT_2 as shown in P-V diagram . Then the ratio of VaVb will be

View SolutionTwo different adiabatic curves for the same gas intersect two isothermals at T_(1), and T_(2) as shown in P-V diagram, (figure). How does the ratio (V_(a)//V_(d)) compare with the ratio (V_(b)//V_(c)) ?
View SolutionA monatomic ideal gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate heat absorbed or released by the gas in process B→C
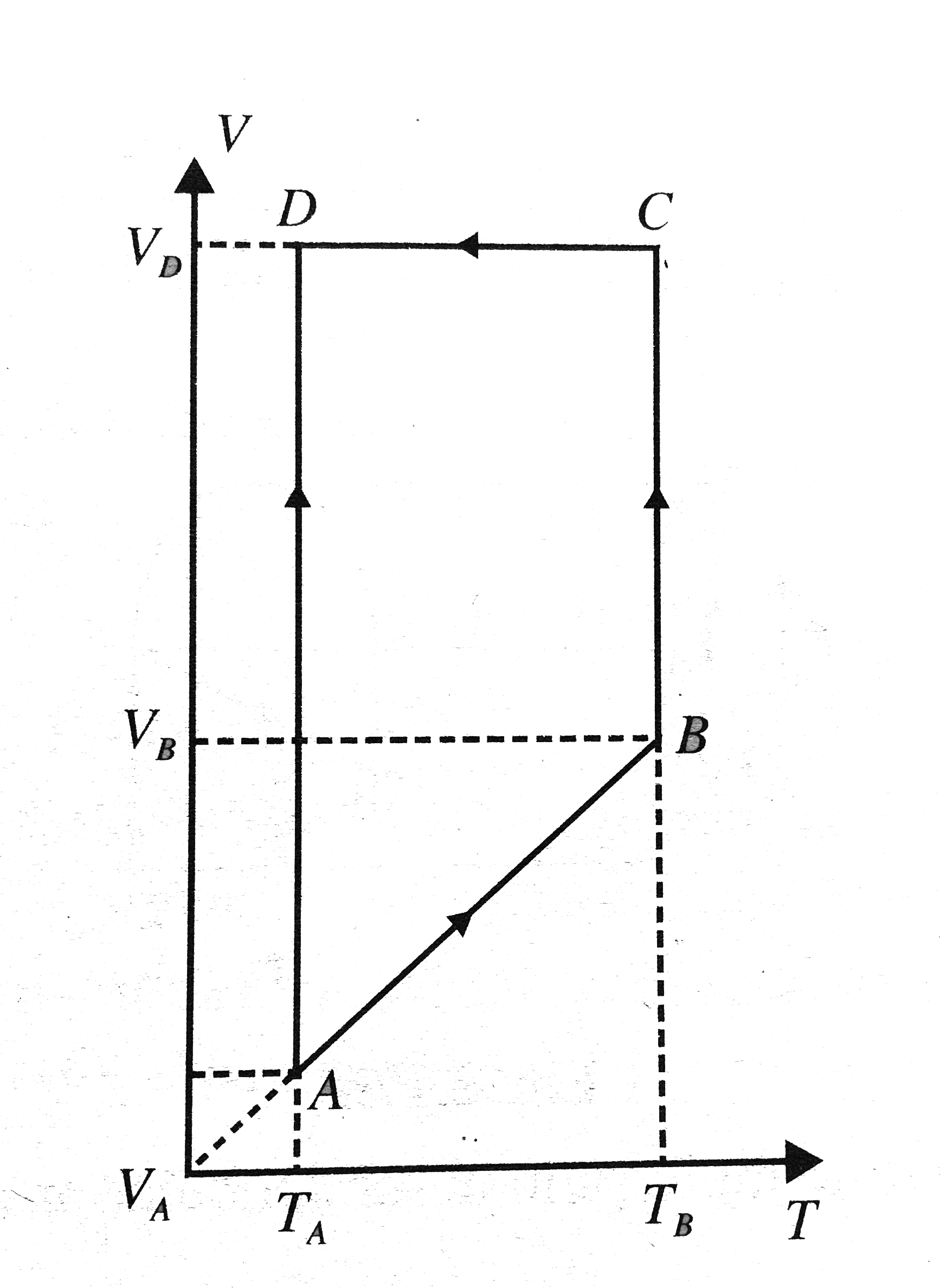
View Solution

