Two different ideal diatomic gases A and B are initially in the same state. A and B are then expanded to same final volume through adiabatic and isothermal process respectively. If PA,PB and TA,TB represents the final pressure and temperature of A and B respectively then.
PA<PB and TA<TB
PA>PB and TA>TB
PA>PB and TA<TB
PA<PB and TA>TB
The correct Answer is:A
To solve the problem, we need to analyze the behavior of two different ideal diatomic gases, A and B, during their respective processes: adiabatic expansion for gas A and isothermal expansion for gas B.
1. Understand the Initial Conditions:
Both gases A and B are initially in the same state, meaning they have the same initial pressure (P₀), volume (V₀), and temperature (T₀).
2. Identify the Processes:
- Gas A undergoes an adiabatic expansion.
- Gas B undergoes an isothermal expansion.
3. Final Volume:
Both gases are expanded to the same final volume (V_f).
4. Analyze the Adiabatic Process (Gas A):
For an ideal gas undergoing adiabatic expansion, the relationship between pressure, volume, and temperature is given by:
PfVγf=P0Vγ0
where γ (gamma) is the heat capacity ratio (C_p/C_v) for diatomic gases, which is approximately 1.4. Since the volume increases, the final pressure (P_A) will be lower than the initial pressure (P_0).
5. Analyze the Isothermal Process (Gas B):
For an ideal gas undergoing isothermal expansion, the pressure and volume relationship is given by:
PfVf=P0V0
Since the temperature remains constant during an isothermal process, the final pressure (P_B) will also be lower than the initial pressure, but the decrease will be less pronounced than in the adiabatic case.
6. Comparison of Final Pressures:
Since gas B is expanded isothermally and gas A is expanded adiabatically, the pressure of gas B (P_B) will be greater than the pressure of gas A (P_A) after expansion:
PB>PA
7. Determine Final Temperatures:
- For gas A (adiabatic), the temperature decreases as it expands, leading to a final temperature (T_A) that is lower than the initial temperature (T_0).
- For gas B (isothermal), the temperature remains constant, so the final temperature (T_B) is equal to the initial temperature (T_0).
8. Final Comparison:
- Since PB>PA and TB=T0 while TA<T0, we conclude:
PB>PAandTB>TA
Conclusion:
The final result is that the pressure of gas B is greater than that of gas A, and the temperature of gas B is greater than that of gas A.
Both gases A and B are initially in the same state, meaning they have the same initial pressure (P₀), volume (V₀), and temperature (T₀).
- Gas A undergoes an adiabatic expansion.
- Gas B undergoes an isothermal expansion.
Both gases are expanded to the same final volume (V_f).
For an ideal gas undergoing adiabatic expansion, the relationship between pressure, volume, and temperature is given by:
PfVγf=P0Vγ0
where γ (gamma) is the heat capacity ratio (C_p/C_v) for diatomic gases, which is approximately 1.4. Since the volume increases, the final pressure (P_A) will be lower than the initial pressure (P_0).
For an ideal gas undergoing isothermal expansion, the pressure and volume relationship is given by:
PfVf=P0V0
Since the temperature remains constant during an isothermal process, the final pressure (P_B) will also be lower than the initial pressure, but the decrease will be less pronounced than in the adiabatic case.
Since gas B is expanded isothermally and gas A is expanded adiabatically, the pressure of gas B (P_B) will be greater than the pressure of gas A (P_A) after expansion:
PB>PA
- For gas A (adiabatic), the temperature decreases as it expands, leading to a final temperature (T_A) that is lower than the initial temperature (T_0).
- For gas B (isothermal), the temperature remains constant, so the final temperature (T_B) is equal to the initial temperature (T_0).
- Since PB>PA and TB=T0 while TA<T0, we conclude:
PB>PAandTB>TA
The final result is that the pressure of gas B is greater than that of gas A, and the temperature of gas B is greater than that of gas A.
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Multiple Corrects
28 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Assertion-Reasoning
6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Subjective
22 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Single correct anwer type
14 Videos
Similar Questions
For an ideal gas
View SolutionStatement I: A quasi -static process is so called bacause it is a sudden and large change of the system.
Statement II: An adiabatic process is not quasi- static because it is a sudden and large change of the system.
View SolutionFive grams of helium having rms speed of molecules 1000m/s and 24g of oxygen having rms speed of 1000m/s are introduced into a thermally isolated vessel. Find the rms speeds of helium and oxygen individually when thermal equilibrium is attained. Neglect the heat capacity of vessel.
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
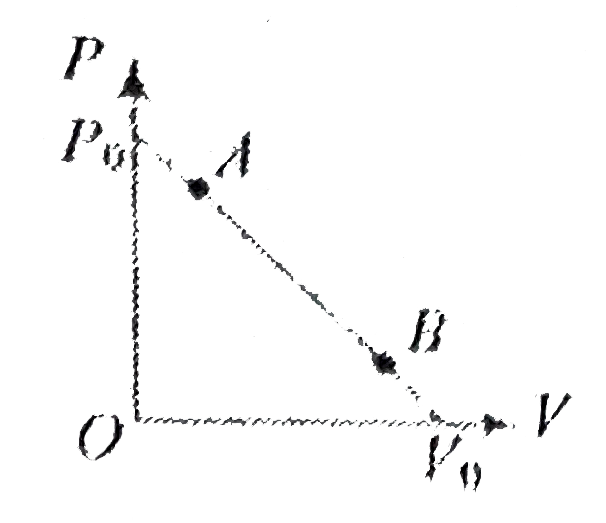
The absolute temperature T versus volume V for the given process is
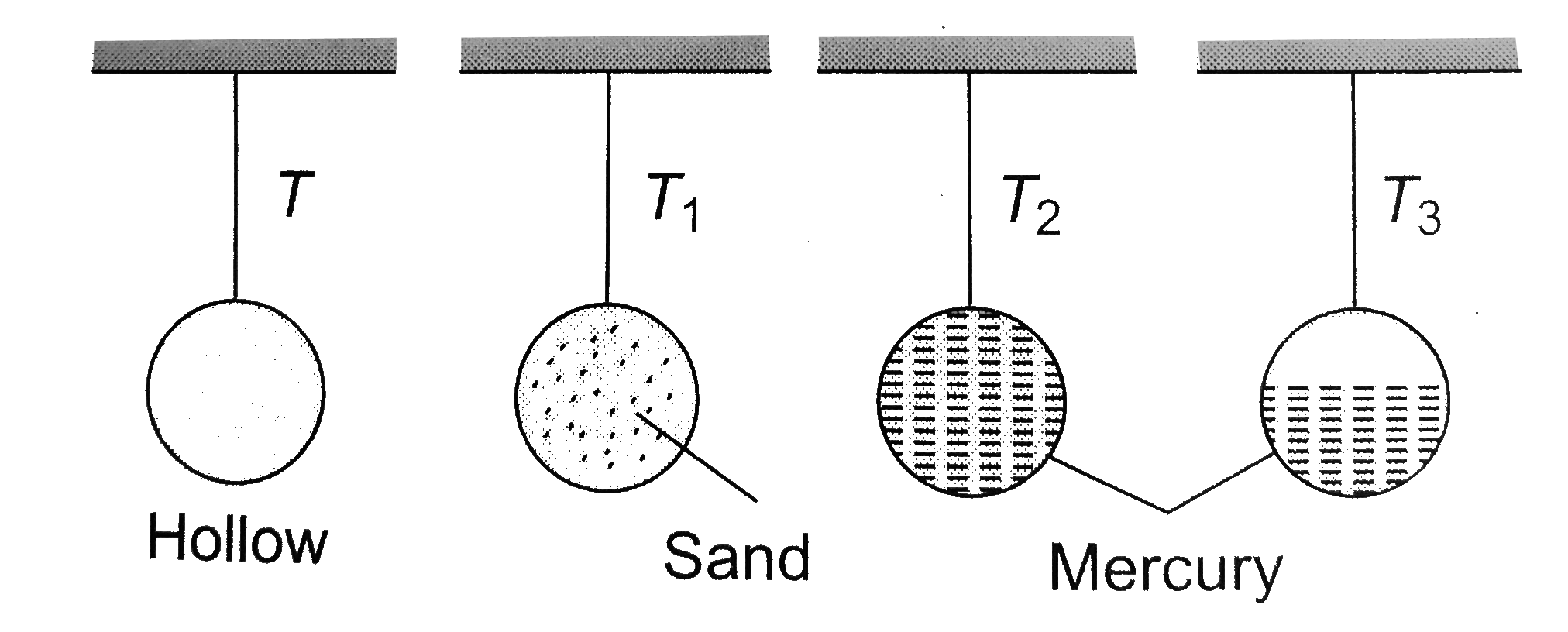
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
View SolutionTwo samples A and B are initaially kept in the same state. The sample A is expanded through an adiabatic process and the sample B through an isothermal process. The final volumes of the samples are the same . The final pressures in A and B are p_A and P_B respectively.
View SolutionTwo different ideal diatomic gases A and B are initially in the same state. A and B are then expanded to same final volume through adiabatic and isothermal process respectively. If P_(A), P_(B) and T_(A), T_(B) represents the final pressure and temperature of A and B respectively then.
View SolutionTwo gases have the same initial pressure, volume and temperature. They expand to the same final volume, one adiabatically and the other isothermally
View SolutionTwo samples A and B are initially kept in the same state. The sample B through an isothermal process. The final volumes of the samples are the same
(a) Compare final temperatures, final pressures and work doen in A and B.
(b) Repeat the part (i), If samples are compressed to same final volume.
View SolutionTwo samples 1 and 2 are initially kept in the same state. The sample 1 is expanded through an isothermal process where as sample 2 through an adiabatic process upto the same final volume. The final temperature in process 1 and 2 are T_(1) and T_(2) respectively, then
View SolutionTwo identical vessels A and B with frictionless pistons contain the same ideal gas at the same temperature and the same volume V. The masses of gaş in A and B are m_(A) and m_(B) respectively. The gases are allowed to expand isothermally to the same final volume 2 V. The change in pressures of the gas in A and B are found to be Deltap and 1.5 Deltap respectively. Then
View SolutionAn ideal gas with pressure P, volume V and temperature T is expanded isothermally to a volume 2V and a final pressure P_(1) . The same gas is expanded adiabatically to a volume 2V, the final final pressure is P_(A) . In terms of the ratio of the two specific heats for the gas gamma , the ratio P_(I)//P_(A) is
View SolutionTwo gases have the same initial pressure, volume and temperature. They expand to the same final volume, one adiabatically and the other isothermally
View SolutionTwo gases have the same initial pressure, volume and temperature. They expands to the same final volume, one adiabatically and the other isothermally.
View SolutionTwo gases have the same initial pressure, volume and temperature. They expand to the same final volume, one adiabatically and the other isothermally.
View Solution

