What is the approximate specific latent heat of melting of ice? State why for cooling botiled soft drinks, ice at0∘C is better than the same mass of iced-water at 0∘C
The approximate specific latent heat of melting of ice is 336×100Jkg−1 1 kg ice at 0∘C takes 336×103J heat to convert into 1 kg iced-water at 0∘C , so it is better for cooling the bottled soft drinks
Topper's Solved these Questions
Similar Questions
The diagram alongside shows a uniform metre rule of weight 100 gf being balanced on a knife edge placed at the 40 cm mark, by suspending a weight w gf at the mark 20 cm, find:
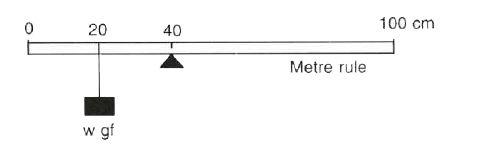
the value of w.
View SolutionName the machine seen in the figure using the principle of echo.

View SolutionChoose the correct statement with respect to Refraction of light
View SolutionA small quantity mass m, of water at a temperature θ(in ∘C) is poured on to a larger mass M of ice which is at its melting point. If c is the specific heat capacity of water and L the specific heat capacity of water and L the specific latent heat of fusion of ice, then the mass of ice melted is give by
View SolutionCalculate change in entropy when 5g of pure ice melts to form water at 0∘C Given latent heat of ice is 80cal/g at 0∘C.
View SolutionCalculate the amount of heat required to convert5g of ice of 0∘C into water at 0∘C.
( Specific latent heat of fusion of ice 80cal/g )
View SolutionCalculate the amount of heat required to convert 80g of ice at 0∘C into water at the same temperature . ( Specific latent heat of fusion of ice =80cal/g )
View Solution1 किग्रा बर्फ का ताप −5∘C है। सम्पूर्ण बर्फ का पिघलाने के लिए इसे कितनी ऊष्मा की आवश्यकता होगी? दिया है- बर्फ की विशिष्ट ऊष्मा = 0.5 कैलोरी/ग्राम ^∘C , तथा बर्फ के गलन की गुप्त ऊष्मा = 80 कैलोरी/ग्राम।
View Solutionक्या कारण है कि शर्बत को ठण्डा करने के लिए 0∘C के जल की अपेक्षा 0∘C की बर्फ अधिक श्रेयस्कर है ?
View Solution0∘C पर 1 किग्रा बर्फ के 0∘C जल में पिघलने पर इसकी एन्ट्रॉपी में परिवर्तन होगा ( बर्फ कि गुप्त ऊष्मा = 80 कैलोरी/.gm ) है -
View SolutionA small quantity mass m, of water at a temperature theta ("in " ^(@)C) is poured on to a larger mass M of ice which is at its melting point. If c is the specific heat capacity of water and L the specific heat capacity of water and L the specific latent heat of fusion of ice, then the mass of ice melted is give by
View SolutionStatement given below is incorrect. Write the correct statement: Water at 0^(@)C cools a soft drink bottles better than ice at 0^(@)C .
View SolutionExplain the following:
Why do soft drink bottles cool better in ice at 0∘C than water at 0∘C?
View SolutionSoft drinks are cooled by placing them in ice rather than water at 0∘C. Why?
View SolutionWhat is the approximate specific latent heat of melting of ice? State why for cooling botiled soft drinks, ice at0∘C is better than the same mass of iced-water at 0∘C
View SolutionWhen 1 g of ice at 0∘C melts to form 1 g of water at 0∘C then, is the latent heat absorbed by the ice or given out by it?
View Solution