What is the relationship between heat capacity and specific heat capacity of a substance ?
Step by step video solution for What is the relationship between heat capacity and specific heat capacity of a substance ? by Physics experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
The figure below shows a simple pendulum of mass 200 g. It is displaced from the mean position A to the extreme position B. The potential energy at the position A is zero. At the position B the pendulum bob is raised by 5 m.
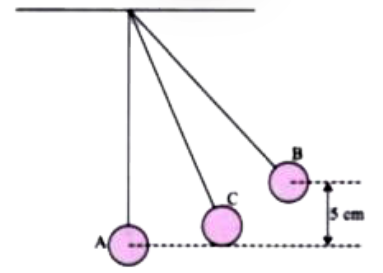
(i) What is the potential energy of the pendulum at the position B?
(ii) What is the total mechanical energy at point C?
(iii) What is the speed of the bob at the position A when released from B ?
(Take g = 10ms−2 and there is no loss of energy.)
View SolutionChoose the correct statement with respect to critical angle
View SolutionShow that the sum of kinetic energy and potential energy (ie, total mechanical energy) is always conserved in the case of a freely falling body under gravity (with air resistance neglected from a height by finding it when (i) the body is at the top c) the body has fallen a distance in the body has reached the ground.
View SolutionDefine specific heat capacity .
View Solutionकिसी पदार्थ की विशिष्ट ऊष्मा धारिता (specific heat capacity) क्या है?
View Solutionकिसी पदार्थ की विशिष्ट ऊष्मा धारिता से क्या तात्पर्य है?
View Solutionकिसी पदार्थ की विशिष्ट ऊष्मा धारिता, ऊष्मा धारिता तथा मोलर विशिष्ट ऊष्मा धारिता से आप क्या समझते है?
View SolutionWhat is meant by specific heat capacity? How will you prove experimentally that different substances have different specific heat capacities?
View SolutionWhat is mean by specific heat capacity ? [OR Define specific heat capacity.] How will you prove experimentally that different substances have different specific heat capacities ?
View SolutionFind the difference between heat capacity and specific heat capacity of a substance.
View SolutionWhat is meant by specific heat capacity? How will you prove experimentally that different substances have different specific heat capacities?
View SolutionDistinguish between the spetific heat capacity and the molar specific heat capacity of a substance
View SolutionSpecific heat capacity of a substance is x cal⋅g−1⋅∘C−1. Specific heat capacity of 100g of that substance will be _____.
View SolutionDistinguish between heat capacity and specific heat capacity.
View SolutionWhat is meant by specific heat capacity? How will you prove experimentally that different substances have different specific heat capacities?
View SolutionWhat is the difference between (i) heat capacity (ii) specific heat capacity and (iii) molar specific heat capacity.
View Solution