Which of the following transitions gives photon of maximum energy?
n=1 to n=2
n=2 to n=1
n=2 to n=6
n=6 to n=2
The correct Answer is:A
Step by step video solution for Which of the following transitions gives photon of maximum energy? by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:dpp 4.2
Explore 15 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:ddp.4.3
Explore 15 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Linked comprehension type
Explore 6 Videos
- ALTERNATING CURRENTBook:CENGAGE PHYSICS ENGLISHChapter:ALTERNATING CURRENTExercise:Single Correct
Explore 10 Videos
- CAPACITOR AND CAPACITANCEBook:CENGAGE PHYSICS ENGLISHChapter:CAPACITOR AND CAPACITANCEExercise:Integer
Explore 5 Videos
Similar Questions
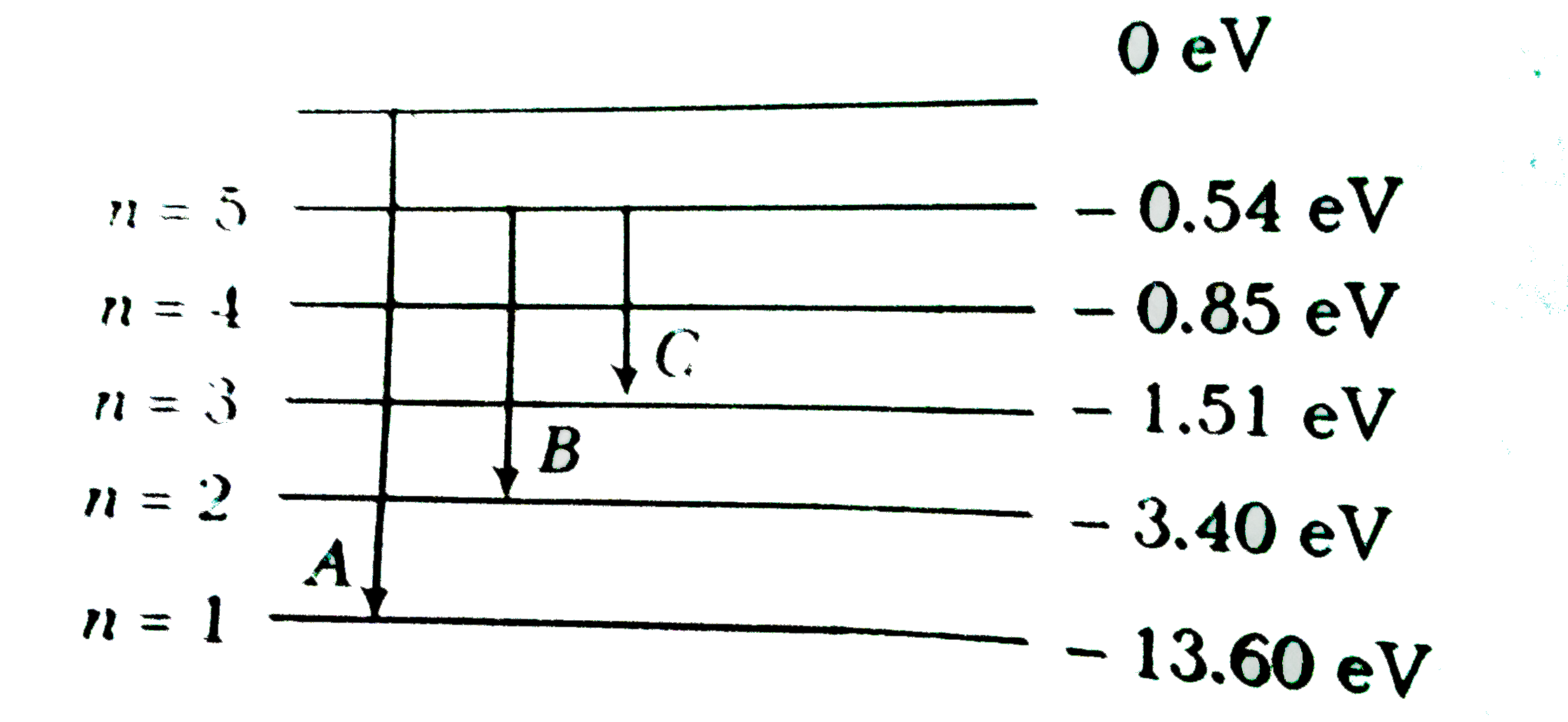
In figure the energy levels of the hydrogen atom have been shown along with some transitions marking A,B,C. The trantions A,B, and C respectively represents
View SolutionThe continuous x - ray spectrum obtained from a Coolidge tube is of the form
View SolutionIn a mixture of H−He+ gas (He+is singly ionized He atom), H atom and He+ ions are excited to their respective first excited state. Subsequently H atoms transfer their total excitation energy to He+ ions (by collisions) Assume that the bohr model of atom is exactly valid.
The quantum number n of the state finally populated in He+ inos is -
View SolutionThe rms current in an AC circuit is 2A. If the wattless current be √3A , what is the power factor of the circuit?
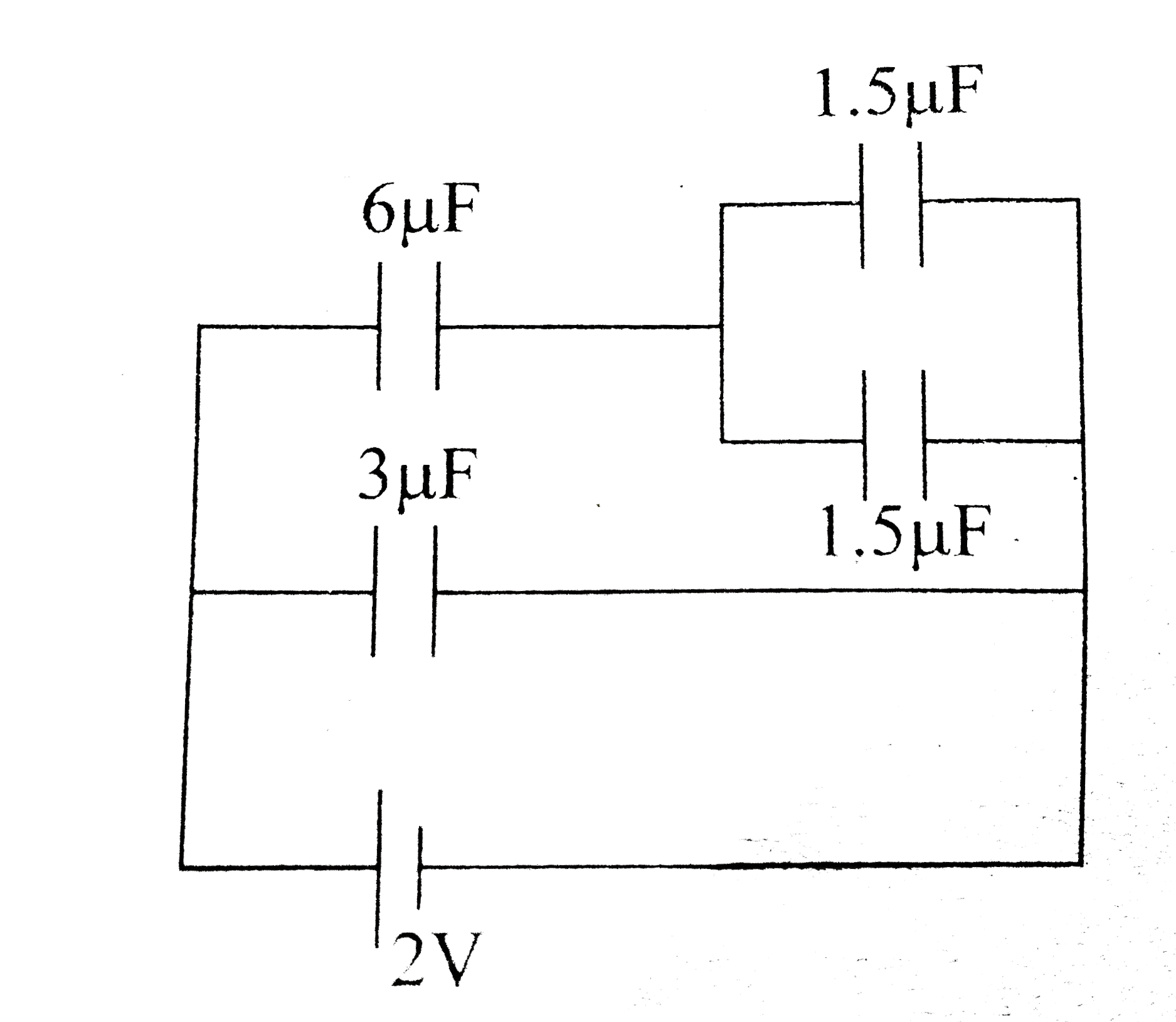
View SolutionEach capacitance shown in is in μF. Find the charge on 6μF.
 .
.
View SolutionWhich of the following transition in a hydrogen atom produces a photon of minimum energy?
View SolutionWhich of the following transition involves maximum amount of energy ?
View SolutionWhich of the following transitions involves maximum amount of energy?
View SolutionWhich of the following transition involves maximum amount of energy ?
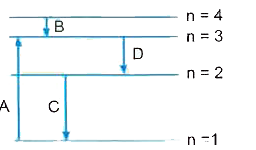
View SolutionThe diagram shows the energy level for an electron in a hydrogen atom. Which transition, shown here, represents the emissions of a photon of maximum energy?
View Solutionएक इलेक्ट्रॉन द्रव्यमान m और एक फोटान की ऊर्जा E समान है। इलेक्ट्रॉन की तरंगदैर्घ्य का फोटॉन से अनुपात है : (c प्रकाश का वेग )
View SolutionWhich of the following transitions will involve the maximum amount of energy?
View Solutionकिस संक्रमण में अधिकतम आवृत्ति का फोटॉन उत्सर्जित होगा
View SolutionWhich of the following transitions gives photon of maximum energy
View SolutionWhich of the following transitions involves maximum amount of energy?
View SolutionWhich of the following transitions involves maximum amount of energy?
View Solution
