Why is there a change in the energy of a gas when its volume changes?Solution in Marathi
Step by step video & image solution for Why is there a change in the energy of a gas when its volume changes? by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
A gas initially at 17∘C issuddenly compressed to 1/8 of its original volume. Find the temperature after compression ? [gamma=53]
View SolutionDistinguish between progressive waves and stationary waves .
View SolutionIf the refractive indices of glass and water with respect to air is 32 and 43 respectively. Calculate the velocity of light in glass and water.From these results calculate the refractive index of glass with respect to water. Given velocity of light in air is 3×108m/s.
View SolutionThe energy change is greatest for a hydrogen atom when its state changes from
View SolutionChange in heat energy of the object when its temperature is changed:
View Solution400 J of work is done on a gas to reduce its volume by compression adiabatically. What is the change in internal energy of the gas?
View SolutionThe ratio of specific heats of a gas is gamma . The change in internal energy of one mole of the gas, when the volume change from V to 2V at constant pressure p is
View SolutionThere is change in volume of an ideal gas when pressure is changed at constant temperature. The change in free energy associated with this can be calculated by which of the following equation ?
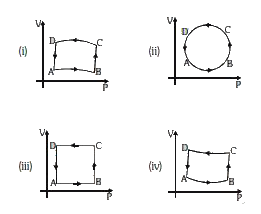
View Solutionचित्र (i) से (iv) में दाब में परिवर्तन के साथ आयतन परिवर्तनदर्शाया गया है। एक गैस को पथ ABCDA के अनुदिश ले जाया जाता है। गैस की आन्तरिक ऊर्जा में परिवर्तन है :
View SolutionThe ratio of specific heats of a gas is γ . The change in internal energy of one mole of gas when the volume changes from v to 2v at constant pressure “p” is
View SolutionThe ratio of specific heats of a gas is γ . The change in internal energy of one mole of gas when the volume changes from v to 2v at constant pressure “p” is
View SolutionThe ratio of specific heats of a gas is γ . The change in internal energy of one mole of gas when the volume changes from v to 2v at constant pressure “p” is
View SolutionInternal energy of an ideal gas changes with change in its
View SolutionChange in heat energy of the object when its temperature is changed:
View SolutionWhen the temperature of 5 mol of a gas is changed from 100^@C to 120^@C keeping the volume constant, the change in internal energy of the gas is 80 J, then find the heat capacity of the gas at constant volume.
View Solution

