Why temperature less than 0∘K is not possible ? Solution in Tamil
Video Solution
Text Solution
According to kinetic theory of gases temperature ∝C2_(RMS) At T=∝C2. At T = 0∘K,C2=0, i.e, molecular motion ceases and kinetic energy becomes zero As kinetic energy. Cannot be negative, therefore temperature below 0∘K is not possible.
|
Similar Questions
Why does the temperature less than zero on absolute scale not possible.
View SolutionIf the both the roots of the quadratic equation x2−2kx+k2+k−5=0 are less than 5, then k
View SolutionExplain why Na is less reactive than K?
View SolutionAt a particular temperature why is the vapour pressure of acetone less than that of ether?
View SolutionName the Cities with the Temperature less than 0∘C?
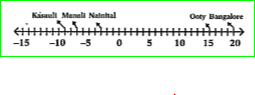
View SolutionName the Cities with The Temperature less than 0∘C?
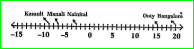
View SolutionWhy is it not possible to cool a gas to '0 K' ?
View SolutionAt a particular temperature why vapour pressure of acetone is less than that of ether.
View Solution
