Work done in converting one gram of ice at −10∘C into steam at 100∘C is
3045 J
6056 J
721 J
6 J
The correct Answer is:A
Work done in converting 1 g of ice at −10∘C to steam at 100∘C
= Heat supplied to raise temperature of 1 g of ice from 10∘C to 0∘C(m×cice×ΔT) + Heat supplied to convert 1 g ice into water at 0∘C(m×Lice)
+ Heat supplied to raise temperature of 1 g of water from
0∘C to 100∘C(m×cwater×ΔT)
+ heat supplied to convert 1 g water into steam at 100∘C[m×Lvapour]
=[m×cice×ΔT]+{m×Lice]+[m×cwater×ΔT]+[m×Lvapour]
=[1×0.5×10]+[1×80]+[1×1×100]+[1×540]=725cal=725×4.2=3045J
Topper's Solved these Questions
- CALORIMETRYBook:CENGAGE PHYSICS ENGLISHChapter:CALORIMETRYExercise:Multiple Correct
Explore 25 Videos
- CALORIMETRYBook:CENGAGE PHYSICS ENGLISHChapter:CALORIMETRYExercise:Comprehension
Explore 30 Videos
- CALORIMETRYBook:CENGAGE PHYSICS ENGLISHChapter:CALORIMETRYExercise:Subjective
Explore 25 Videos
- BASIC MATHEMATICSBook:CENGAGE PHYSICS ENGLISHChapter:BASIC MATHEMATICSExercise:Exercise 2.6
Explore 20 Videos
- CENTRE OF MASSBook:CENGAGE PHYSICS ENGLISHChapter:CENTRE OF MASSExercise:INTEGER_TYPE
Explore 1 Video
Similar Questions
Due to thermal expansion with rise in temperature:
View SolutionA body cools in a surrounding of constant temperature 30∘C Its heat capacity is 2J/∘C. Initial temperature of cooling is valid. The body of mass 1 kg cools to 38∘C in 10 min
When the body temperature has reached 38∘C, it is heated again so that it reaches 40∘C in 10 min. The heat required from a heater by the body is
View SolutionA lead ball at 25∘C is dropped from a height of 2 km. It is heated due to air resistance and it is assumed that all of its kinetic energy is used in increasing the temperature of ball. Find the final temperature of the ball.
View SolutionThe displacement of a particle is given by y=(6t2+3t+4)m, where t is in seconds. Calculate the instantaneous speed of the particle.
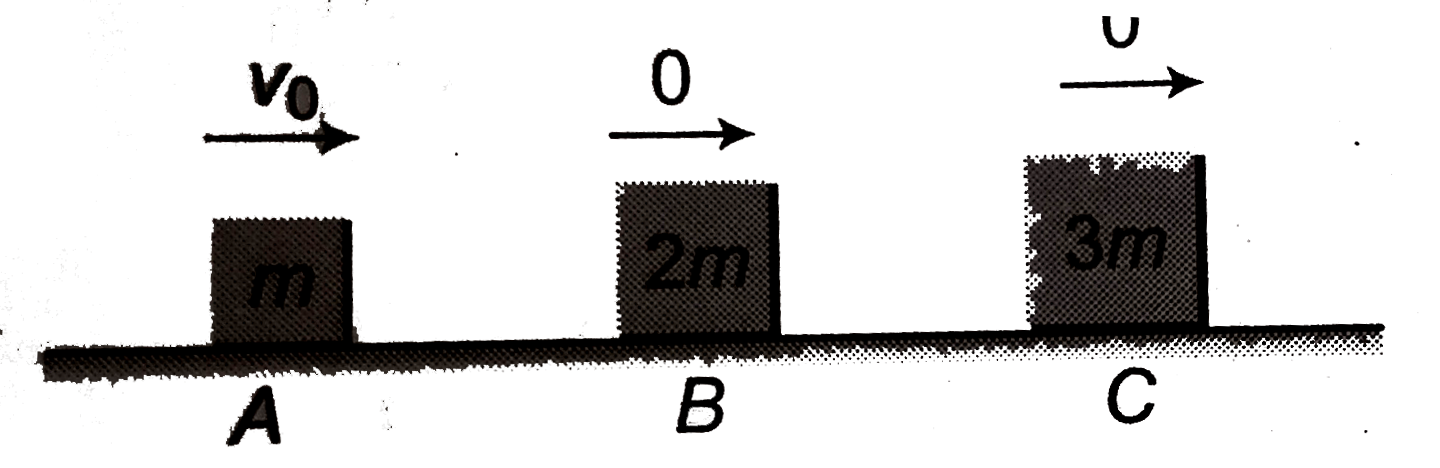
View SolutionThree objects A ,B and C are kept in a straight line on a smooth horizontal surface. These have masses m, 2m and 3m , respectively . The head - on elastic collision takes place between A and B and then B makes completely inelastic collision with C. All motions occur on the same straight line. The final speed of C will be
View SolutionWork done in converting 1 g of ice at -10^@C into steam at 100^@C is
View Solution0∘C ताप वाले 50 ग्राम बर्फ को 100∘C की भाप में बदलने के लिये आवश्यक ऊष्मा की गणना कीजिये।
View Solution−10∘C पर 1 ग्राम बर्फ को 100∘C की भाप में बदलने के लिए कितनी ऊष्मा की आवश्यकता होगी ? बर्फ की विशिष्ट ऊष्मा = 0.5 कैलोरी/ग्राम∘C , बर्फ की गुप्त ऊष्मा = 80 कैलोरी/ग्राम तथा भाप की गुप्त ऊष्मा = 540 कैलोरी/ग्राम।
View SolutionFind amount of heat released if 100 g ice at – 10°C is converted into 120°C, 100 g steam.
View SolutionFind amount of heat released if 100 g ice at – 10°C is converted into 120°C, 100 g steam.
View Solution0∘C के 10 ग्राम बर्फ को 100∘C की भाप में परिवर्तित करने के लिए कितनी ऊष्मा की आवश्यकता होगी ?
बर्फ की गुप्त 80 ऊष्मा कैलोरी/ग्राम |
View SolutionThe amount of work done to convert 1 g ice at 0^(@)C into steam at 100^(@)C is
View Solution0° C Of temperature 1 g Ice 100°C This is the amount of work that needs to be done to convert the temperature into steam
View Solution−10∘C ताप वाली 10 ग्राम बर्फ को 100∘C ताप वाली भाप में परिवर्तित करने के लिए कितने कैलोरी ऊष्मा की आवश्यकता होगी?
View SolutionWork done in converting 1 gm of ice at- 10∘C into steam at 100∘C is
View Solution10∘C की एक ग्राम बर्फ को 100∘C की 1 ग्राम भाप के साथ मिलाने पर बने जल का द्रव्यमान ज्ञात कीजिये |
View Solution
