Complete Solutions to PART - D of chapter ANNUAL EXAM QUESTION PAPER MARCH - 2017 of Class 12 book with complete answers and questions
| Class 12 | CHEMISTRY |
| Chapter | ANNUAL EXAM QUESTION PAPER MARCH - 2017 |
| SUNSTAR PUBLICATION | PART - D |
| Number Of Questions | 28 |
| Academic Year | 2023-24 |
Calculate the packing efficiency in a simple cubic lattice.
View SolutionAn element having atomic mass 63.1 g/mol has face centred cubic unit cell with edge length `3.608xx10^(-8)cm`. Calculate the density of unit cell. [Given : `NA=6.022xx1023" atoms/mol"`].
View Solution1.0 g of non - electrolyte solute dissolved in 50 g of benzene lowered the freezing point of benzene by 0.4 K. Find the molar mass of the solute. [Given : Freezing point depression constant of benzene = 5.12 K. kg mol].
View SolutionHow solubility of a gas in liquid changes with increase in temperature?
View SolutionThe electrode potential for the Danicell given below is 1.1V.
`Zn(s)|Zn^(2+)(aq)||Cu^(2+)(aq)|Cu(s)`
Write overall cell reaction and calculate the standard Gibb's energy for the reaction. `[F=96487c//mol]`.
View SolutionMention any two factors which affects the conductivity of electrolytic solution.
View SolutionDerive an integrated rate equation for the rate constant of a zero order reaction.
View SolutionWrite
i) Arrhenius equation.
ii) The formula to calculate half life. Period of zero order reaction.
View Solutionwrite any two differences between lyophilic and lyophobic colloids .
View SolutionExplain the mechanism of enzyme catalysis.
View SolutionHow does entropy change for adsorption?
View SolutionWrite `SN^(2)` mechanism of the conversion of methyl chloride to methyl alcohol.
View SolutionAldehydes are generally more reactive than ketones towards nucleophillic addition reactions. Give two reasons.
View SolutionWhat is asymmetric carbon atom.
View SolutionExplain the Kolbe's reaction with equation.
View SolutionWrite the mechanism of acid catalysed dehydration of ethanol to ethene.
View SolutionHow benzene is converted into benzaldehyde by Gatterman-Koch reaction.
View SolutionComplete and name the following reaction.
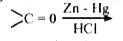
View SolutionWhat is the effect of electron withdrawing group on the acidity of carboxylic acid ?
View SolutionHow aniline is prepared by Hoffmann bromamide degradation reaction? Give equation.
View Solutioni) Write IUPAC name of `CH_(3)CH_(2)NH_(2)`.
ii) Arrange the following amines in the order of their increasing basic strength in aqueous solution.
`(CH_(3))_(3)N_(1), (CH_(3))_(2)NH_(1), CH_(3)NH_(2)`.
View SolutionComplete the following equation.
`C_(6)H_(5)NH_(2)+NaNO_(2)+2HCl overset(273-278K)rarr`
View SolutionWrite Haworth structure for maltose.
View SolutionGive an example for
i) Globular proteins.
ii) Naturally occurring optically inactive amino acid.
View SolutionName the nucleic acid which is responsible for genetic information.
View SolutionExplain the preparation of Buna-N with equation.
View SolutionName the monomer present in the following polymer
i) Poly vinyl chloride. Ii) Natural rubber.
View SolutionGive an example for biodegradable polymer.
View Solution
PART - D questions and complete solutions for chapter ANNUAL EXAM QUESTION PAPER MARCH - 2017 of SUNSTAR CHEMISTRY (KANNADA ENGLISH) of Class 12