From the following statements concerning ideal gas at any given temperature T, select the correct one (s)
The coefficient of volume expansion at constant pressure is the same for all idea gases
The average translational kinetic energy per molecule of oxygen gas is 3kT,k being Boltzmann constant
The mean-free path of molecules increases with increases in the pressure
In a gaseous mixture, the average translational kinetic energy of the molecules of each component is different
The correct Answer is:A
(a) For an ideal gas PV=nRT
⇒ Coefficient of volume expansion
(ΔVΔT)p=nRP=Constant
Similar Questions
From the following statements concerning ideal gas at any given temperature T,select the correct one(s).
View SolutionFrom the following statements concerning ideal gas at any given temperature T, select correct one(s).
View Solutionकिसी ताप T पर आदर्श गैस के लिए निम्न में से सत्य कथन है
View Solutionwhich one of the following graph is not correct for ideal gas ?
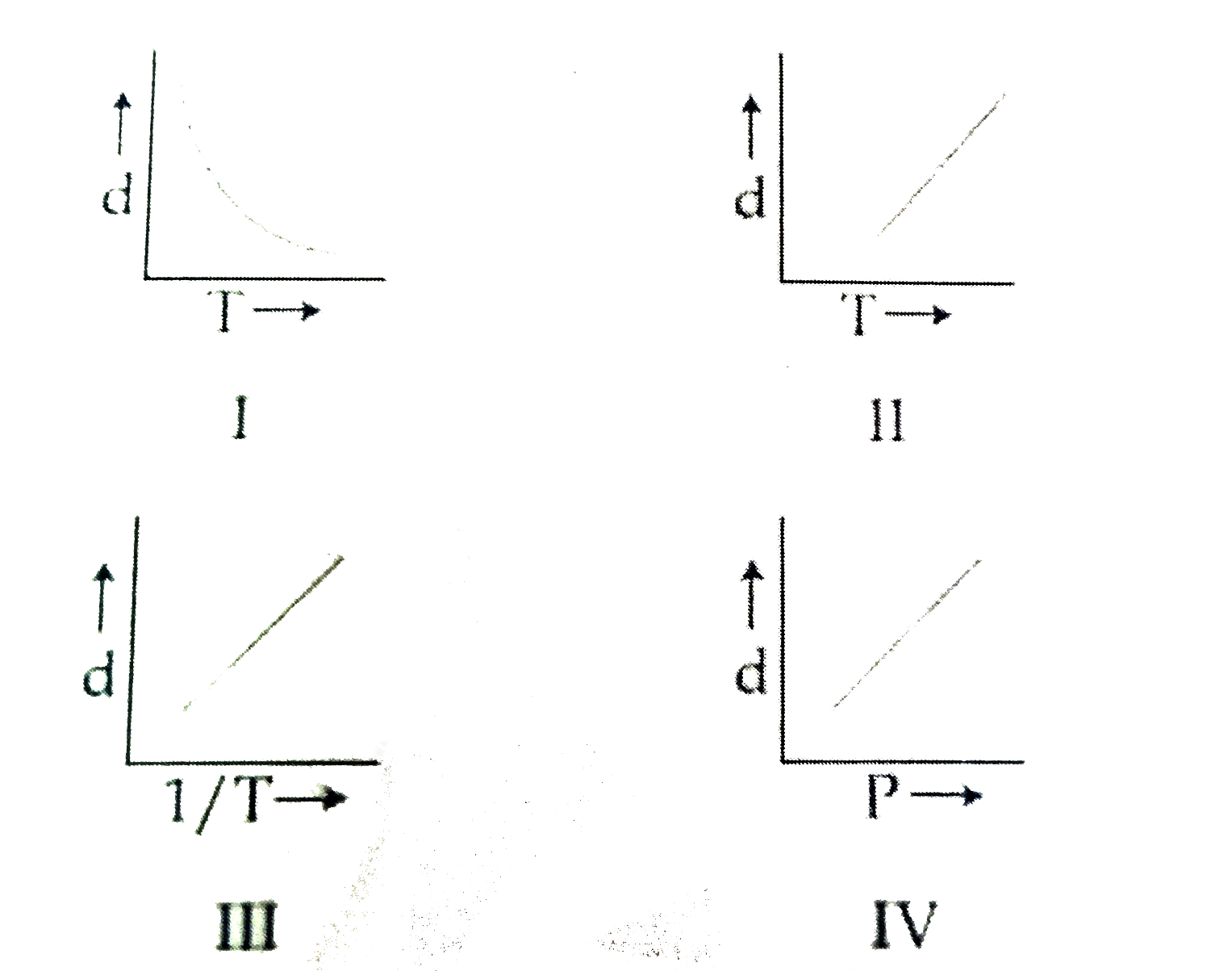
d=density ,P=pressure,T=Temperature
View SolutionFrom the following statements concerning ideal gas at any given temperature T, select the correct one
View SolutionSelect the correct statement for isothermal expansion of an ideal gas
View Solutionकिसी ताप T पर आदर्श गैस के लिए में से असत्य कथन है
View SolutionFrom the following statements concerning ideal gas at any given temperature T, select the correct one (s)
View Solution

