Which of the following is the weakest base ?
A
Ethyl amine
B
Ammonia
C
Dimethyl amine
D
Methyl amine
Video Solution
Text Solution
The correct Answer is:B
Alkyl group (an electron relasing(+I) group) increases electron density, at N-atom hence, basic nature is increases.
In ammonia, no alkyl group in present, so it is least basic.
|
Similar Questions
Knowledge Check
Which of the following is the weakest acid ?
A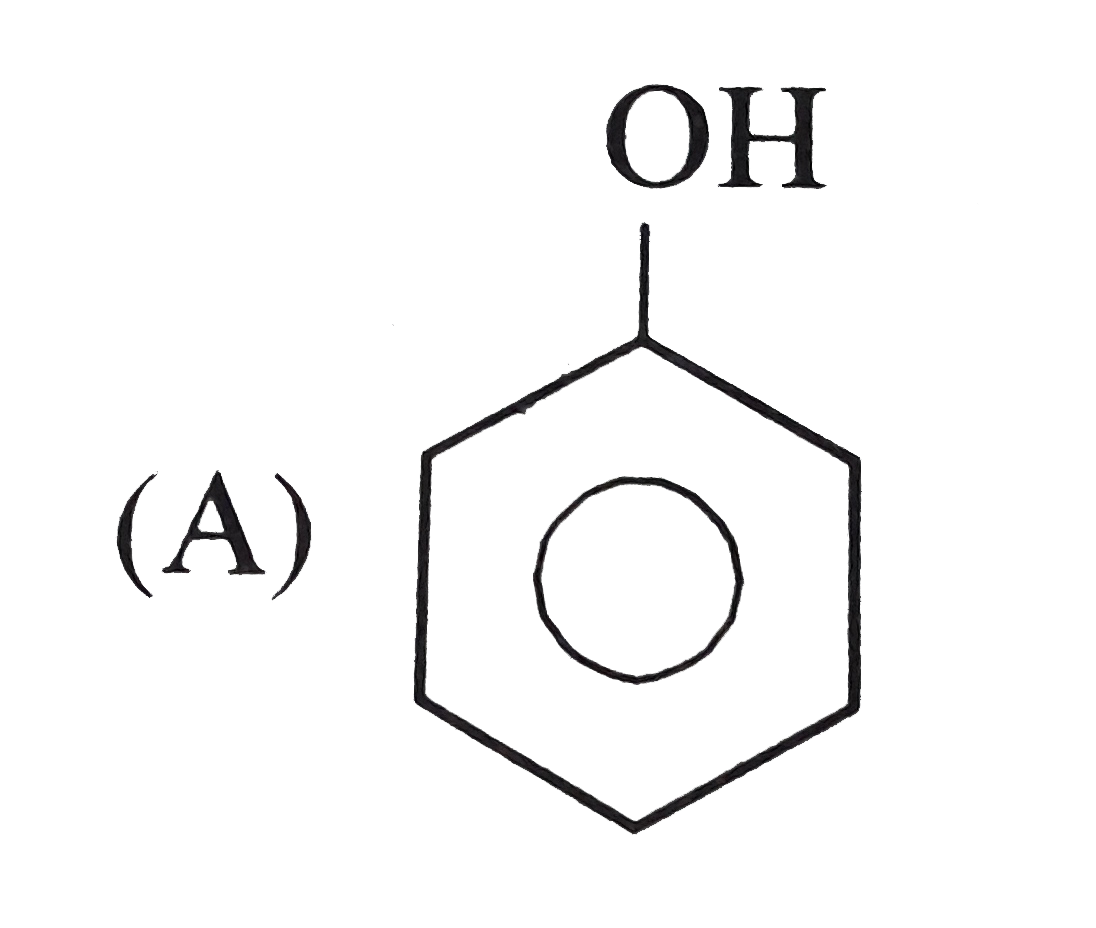 BCH3COOHCHCOOHD
BCH3COOHCHCOOHD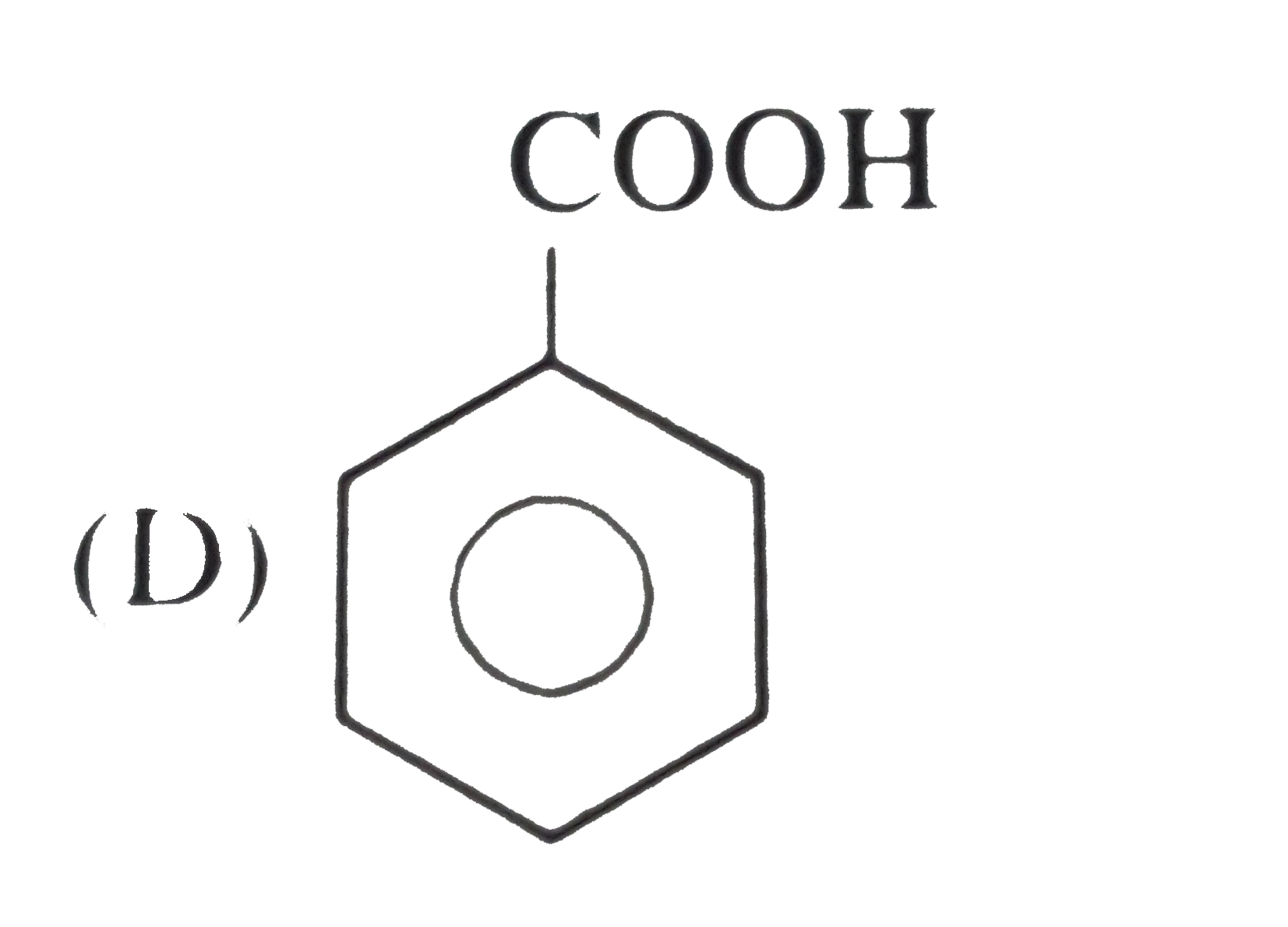
Which of the following is the weakest acid ?
APhenol (Ka=1.3×1010)BHydrocyanic acid (Ka=4.9×10−10)CAcetic acid (Ka=1.8×10−5)DBenzoic acid (Ka=6.5×10−5)Which of the following is the weakest acid ?
AHClBHFCH2SO4DHNO3
Which of the following is the weakest acid
View SolutionWhich one of the following is the weakest base ?
View SolutionWhich one of the following is the weakest base?
View SolutionWhich one of the following is the weakest base in queous medium ?
View SolutionWhich of the following is the weakest acid?
View Solution
