In ......... process , work is done at the expense of internal energy .
isothermal
isochloric
adiabatic
isobaric
The correct Answer is:C
We know that ΔE=Q+W
If heat supplied from the surrounding . Q=0
∴ΔE=W
i.e., work is done at the expense of only internal energy and Q= 0 for adiabatic process .
Similar Questions
In a process, 701J of heat is absorbed by a system and 394J of work is done by the system. What is the change in internal energy for the process?
View SolutionIn a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system.What is the change in internal energy for the process?
View Solution
Knowledge Check
Work done at the expense of internal energy in
AIsothermal processBIsochoric processCAdiabatic processDReversible processIn an adiabatic process 90 J of work is done on the gas. The change in internal energy of the gas is
A−90jB+90jC0 jDDepends on initial temperature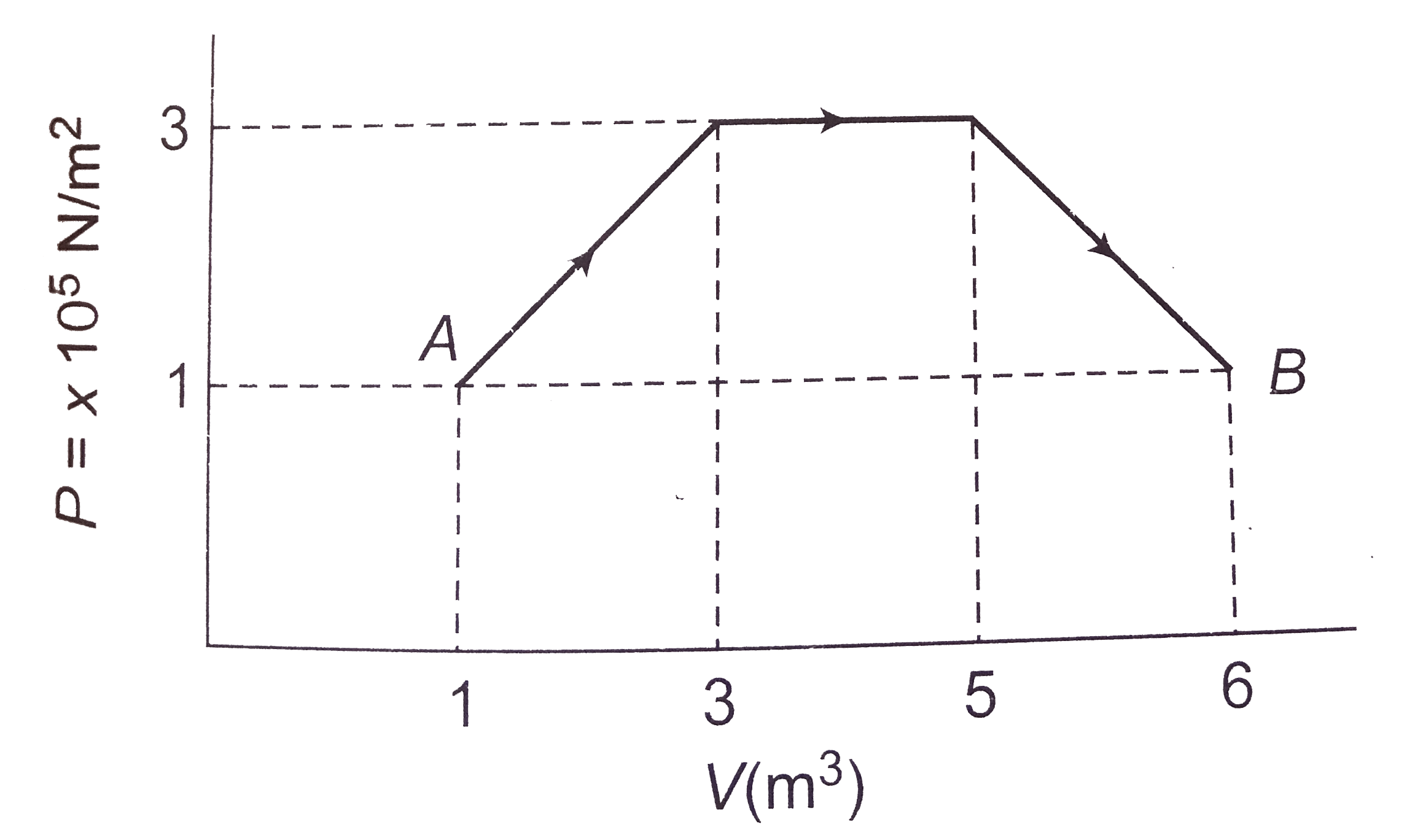
The work done in the process AB isA8×105JB10×105JC7×105JD12×105J
In thermodynamic process, 200 Joules of heat is given to a gas and 100 Joules of work is also done on it. The change in internal energy of the gas is
View SolutionAssertion: In adiabatic process work is independent of path.
Reason: In adiabatic process work done is equal to negative of change in internal energy.
View SolutionIn a cyclic process, work done by the system is
View SolutionIn a cyclic process, work done by the system is
View SolutionIn a process, 701J of heat is absorbed by a system and 394J of work is done by the system. What is the change in internal energy for the process?
View Solution
