What is the action of the following reagents on glucose?
(a) bromine water (b) dilute HNO3
hydroxyl amine
(a) Bromine water : When glucose reacts with bromine water, gluconic acid is formed.
CH2OHGlucose(CHOH)4CHO+[O]Br2/H2O−−−−−→CH2OH(CHOH)4Gluconic acidCOOH
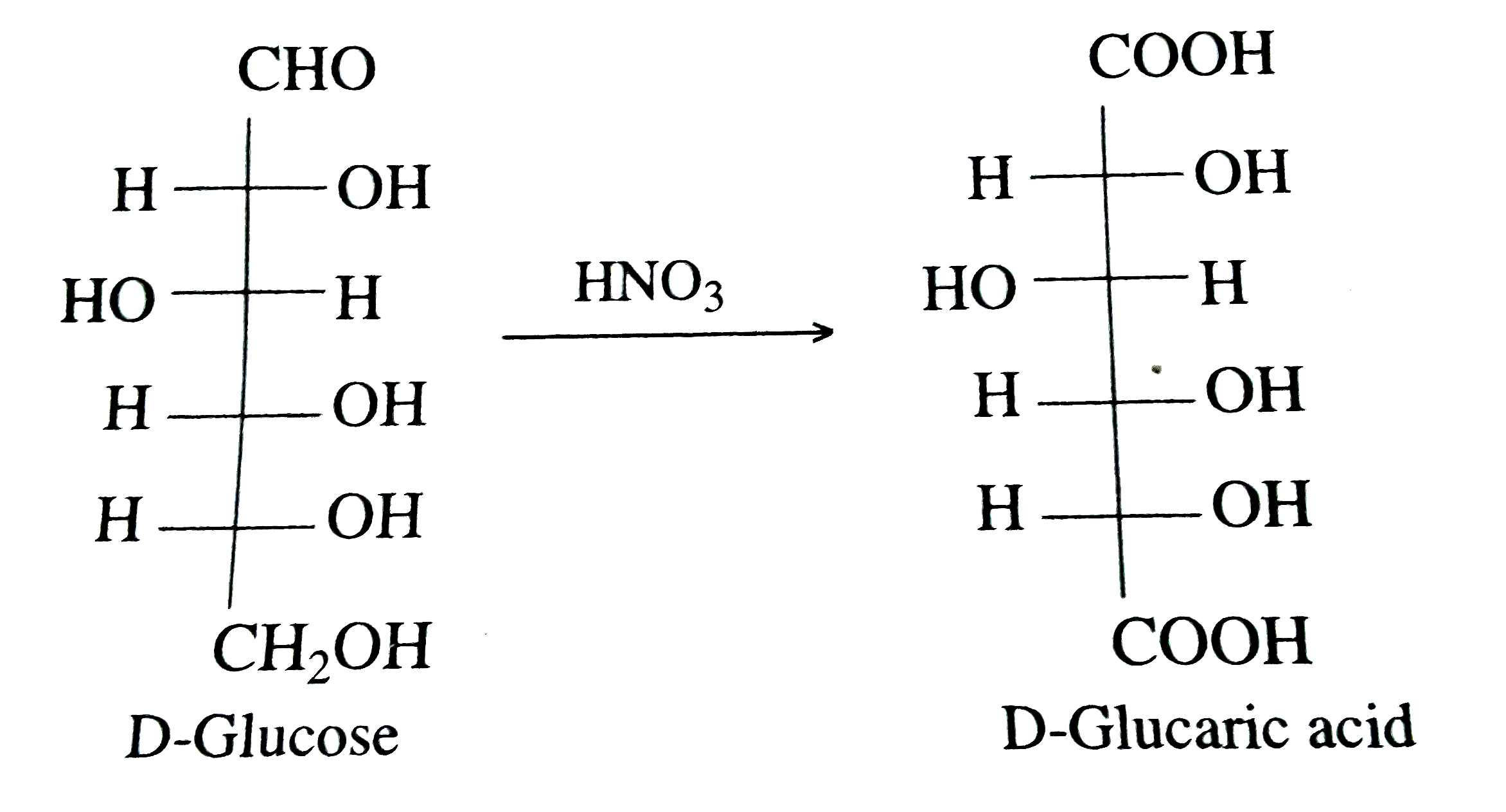
(b) Dilute HNO3 : When D-Glucose is treated with nitric acid, oxidation of D-Glucose takes place. The aldehyde and alcohol group of glucose are ozidized to carboxylic acids forming D-Glucaric acid.

(c ) Hydroxylamine : D (+) glucose reacts with hydroxyl amine and yield an oxime. The structure of the oxime would be:
Similar Questions
What are racemates ?
What is the action of the following reagents on glucose ?
(a) Bromine water (b) Hydroxylamine
View SolutionWhat is the action of following reagents on glucose ?
hydroxylamine
View SolutionWhat is the action of following reagents on glucose ?
acetic anhydride
View SolutionWhat is the action of following reagents on glucose ?
hydrogen iodide
View SolutionDescribe the action of following reagents on glucose:
HI
View SolutionWhat is the action of the following reagents on aniline ?
(a) Bromine water
(b) Acetie anhydride
(c) Hot and conc. sulphuric acid
View SolutionWhat is the action of the following regents on ammonia :
(a) Nessler's reagent
(b) Sodium metal
View SolutionDescribe the action of Hydroxyl amine reagents on glucose.
View SolutionDescribe the action of Bromine water reagents on glucose .
View SolutionWhat is the action of bromine water on (i) glucose (ii) fructose?
View Solution

