Identify the weakest base amongst the following :
A
p-methoxyaniline
B
o-toluidine
C
Benzene-1, 4-diamine
D
4-aminobenzoic acid
Video Solution
Text Solution
The correct Answer is:B
p-methoxyaniline group, donates electrons to the benzene ring thereby increasing group (−OCH3) being the electron donating group, donates electrons to the benzene ring thereby increasing the concentration of electrons on the ring and thus the attack of nucleophile becomes difficult.
|
Similar Questions
Knowledge Check
The weakest base among the following
AI−BCl−CF−DBr−The weakest base among the following is
AC6H5SO−3BC2H5O−CC6H5O−DCH3−CH=CH−CH2−O−Weakest base among the following is:
A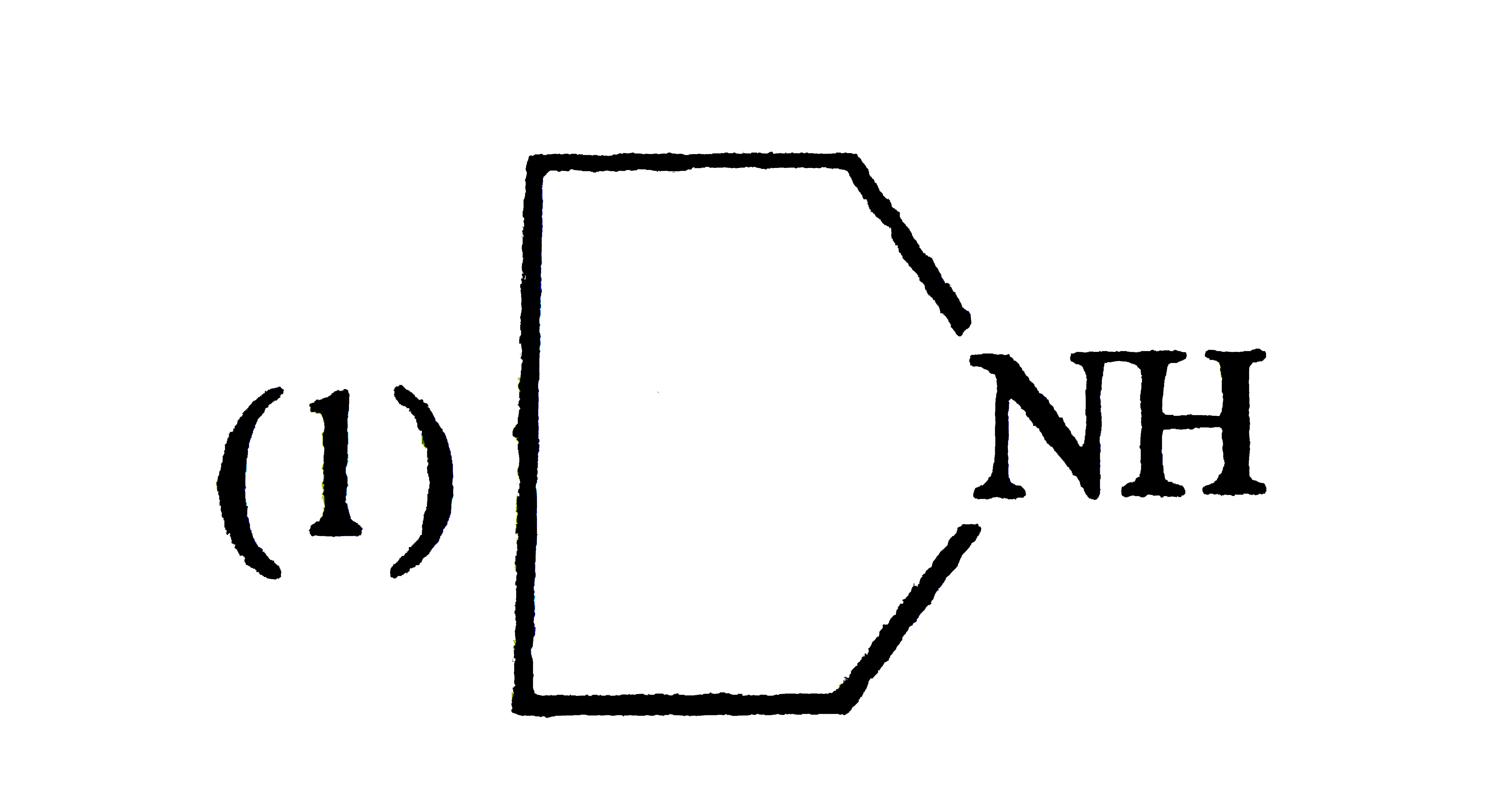 B
B C
C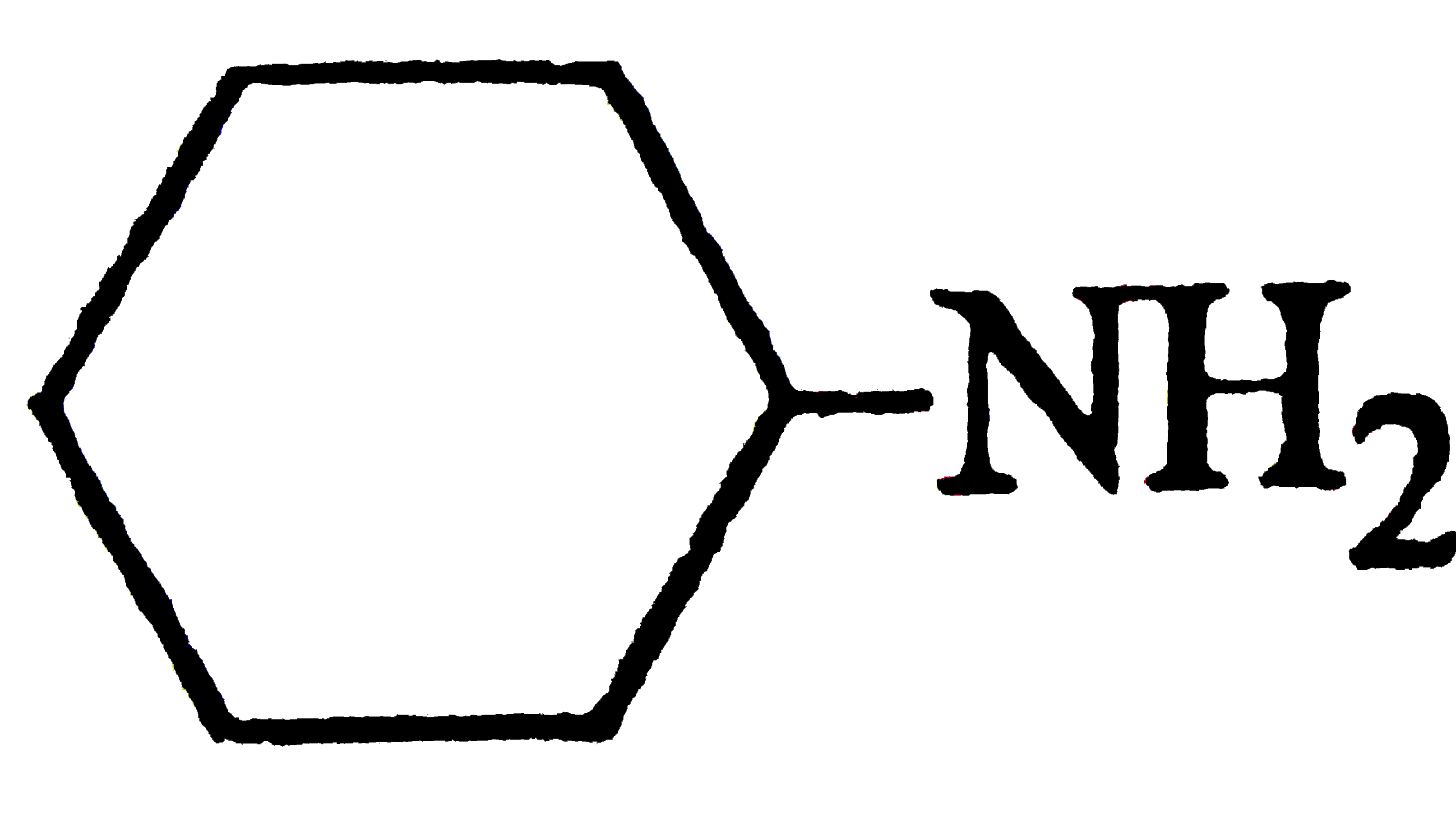 D
D
Identify the strongest acid amongst the followings :
View SolutionIdentify the strongest acid amongst the following
View SolutionIdentify the least stable ion amongst the following .
View SolutionIdentify the least stable ion amongst the following:
View SolutionIdentify the least stable ion amongst the following:
View Solution
