The correct structure of tribromootaoxide.
A
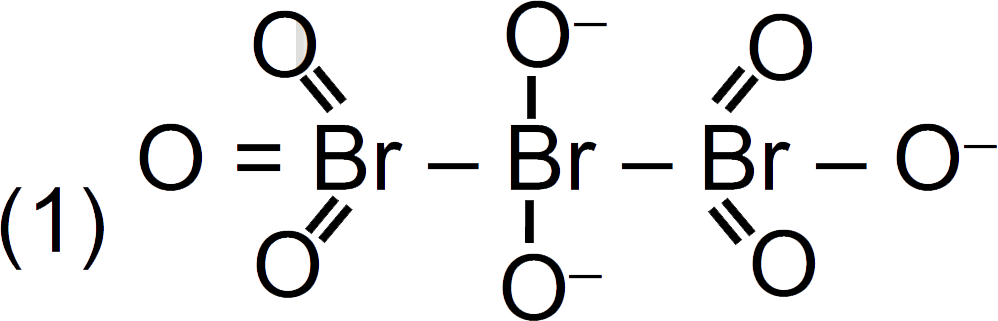
B
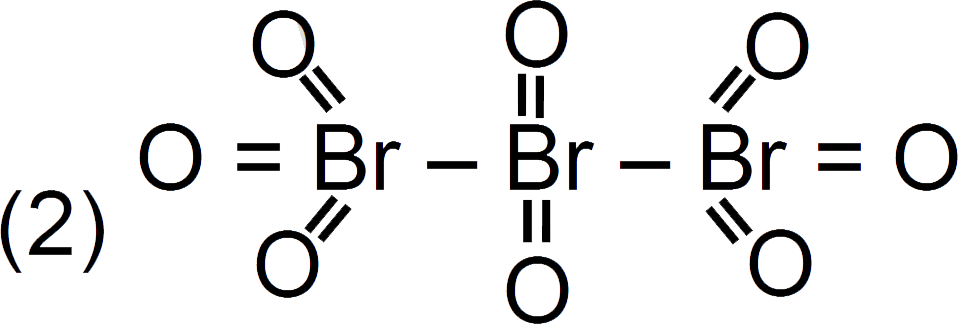
C
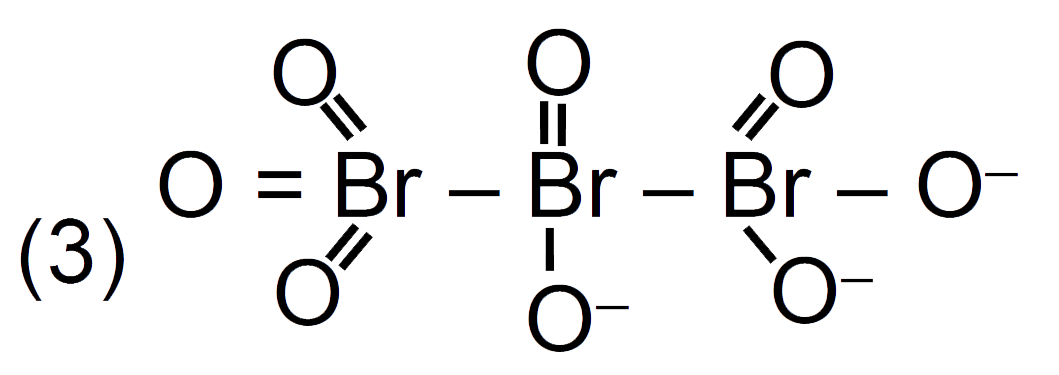
D
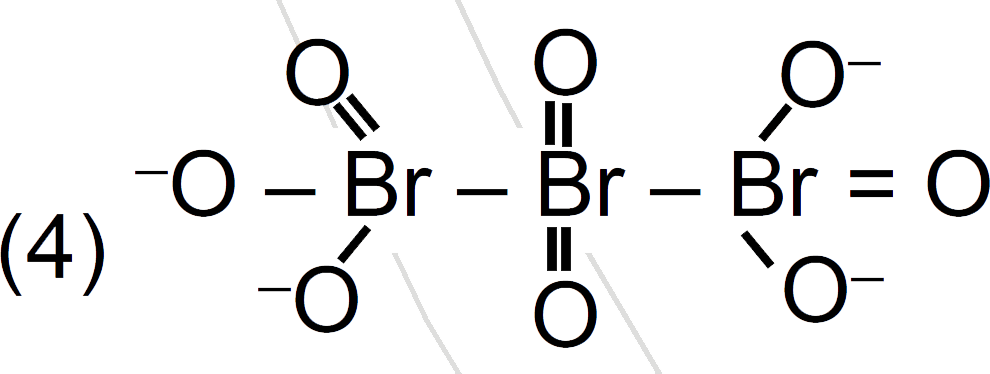
Video Solution
Text Solution
The correct Answer is:B
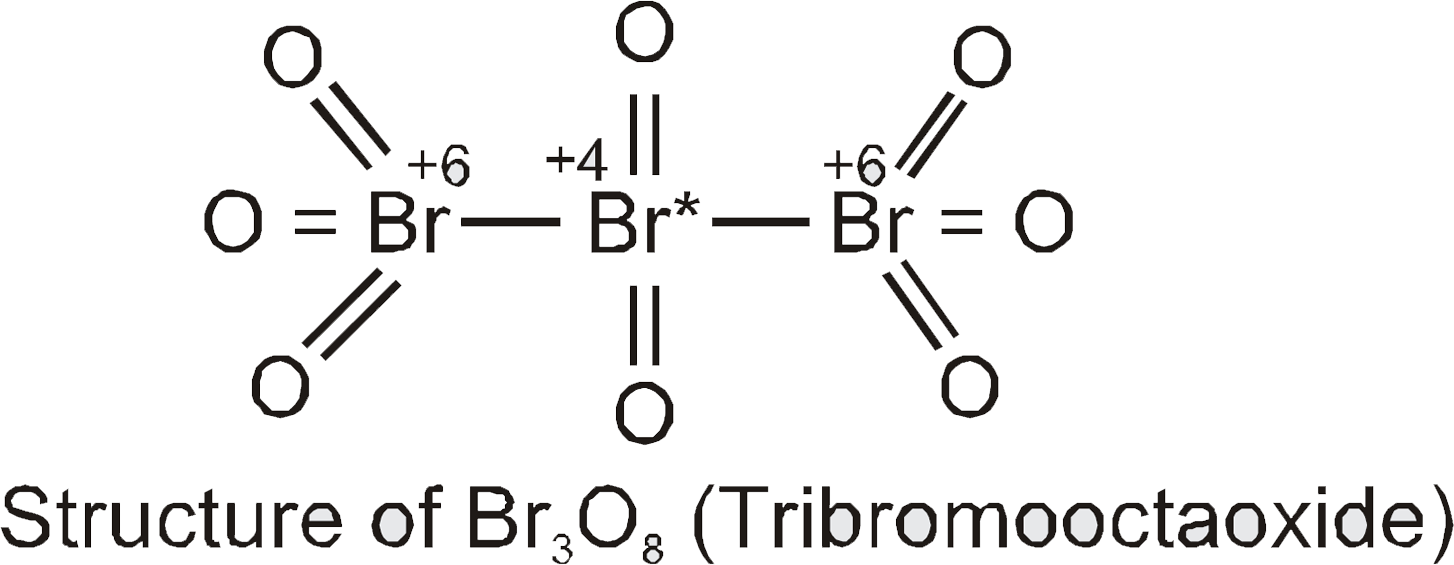
Likewise in Br3O8 each of the two terminal bromine atoms are present in +6 oxidation state and the middle bromide is present in +4 oxidation state. Once again the average, that is different from reality is +16/3
|
Topper's Solved these Questions
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:QUESTION
Explore 87 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQ
Explore 91 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQ
Explore 91 Videos
- METALLURGICAL OPERATIONS Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:METALLURGICAL OPERATIONS Exercise:MCQ
Explore 21 Videos
- NEET 2018Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEET 2018Exercise:QUESTION
Explore 45 Videos
Similar Questions
Knowledge Check
The correct structure of tribromootaoxide.
A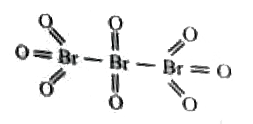 B
B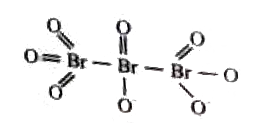 C
C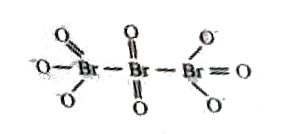 D
D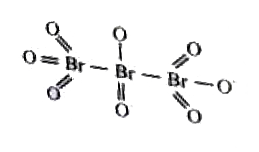
The correct structure of tribromootaoxide.
A B
B C
C D
D
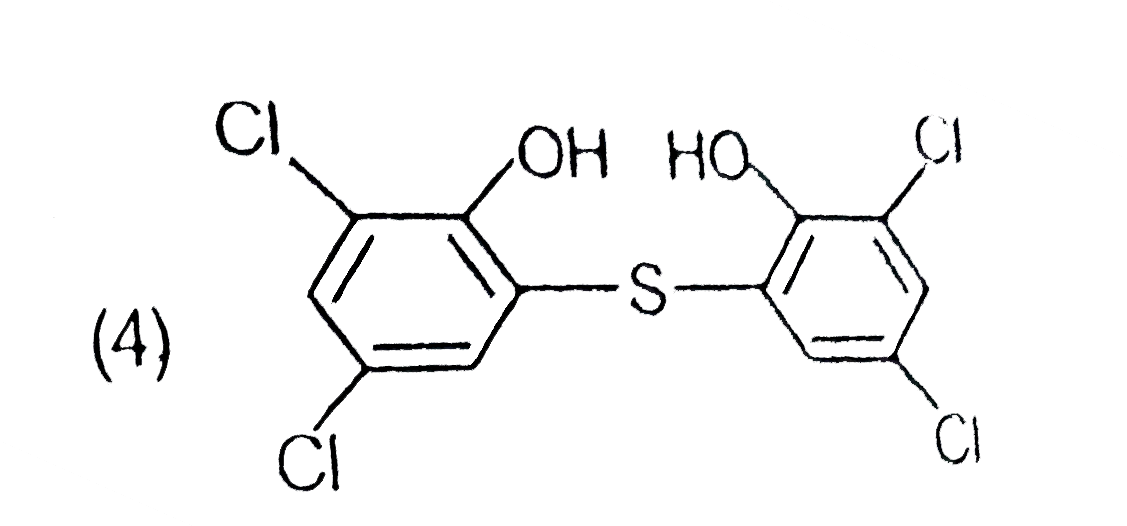
The correct structure of Bithional is
A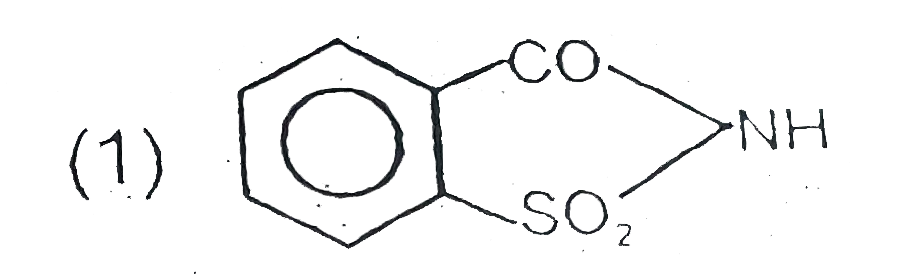 B
B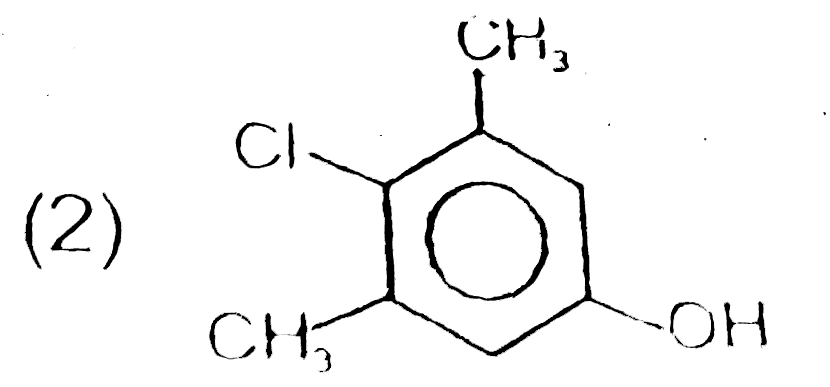 C
C D
D