A mass of gas is first expanded isothermally and then compressed adiabatically to its original volume. What further simplest operation must be performed on the gas to restore it to its original state?
An isobaric cooling to bring its temperature to initial value
An isochoric cooling to bring its pressure to its initial value
An isothermal process to take its pressure to its initial value
An isochoric heating to bring its temperature to initial value
The correct Answer is:B
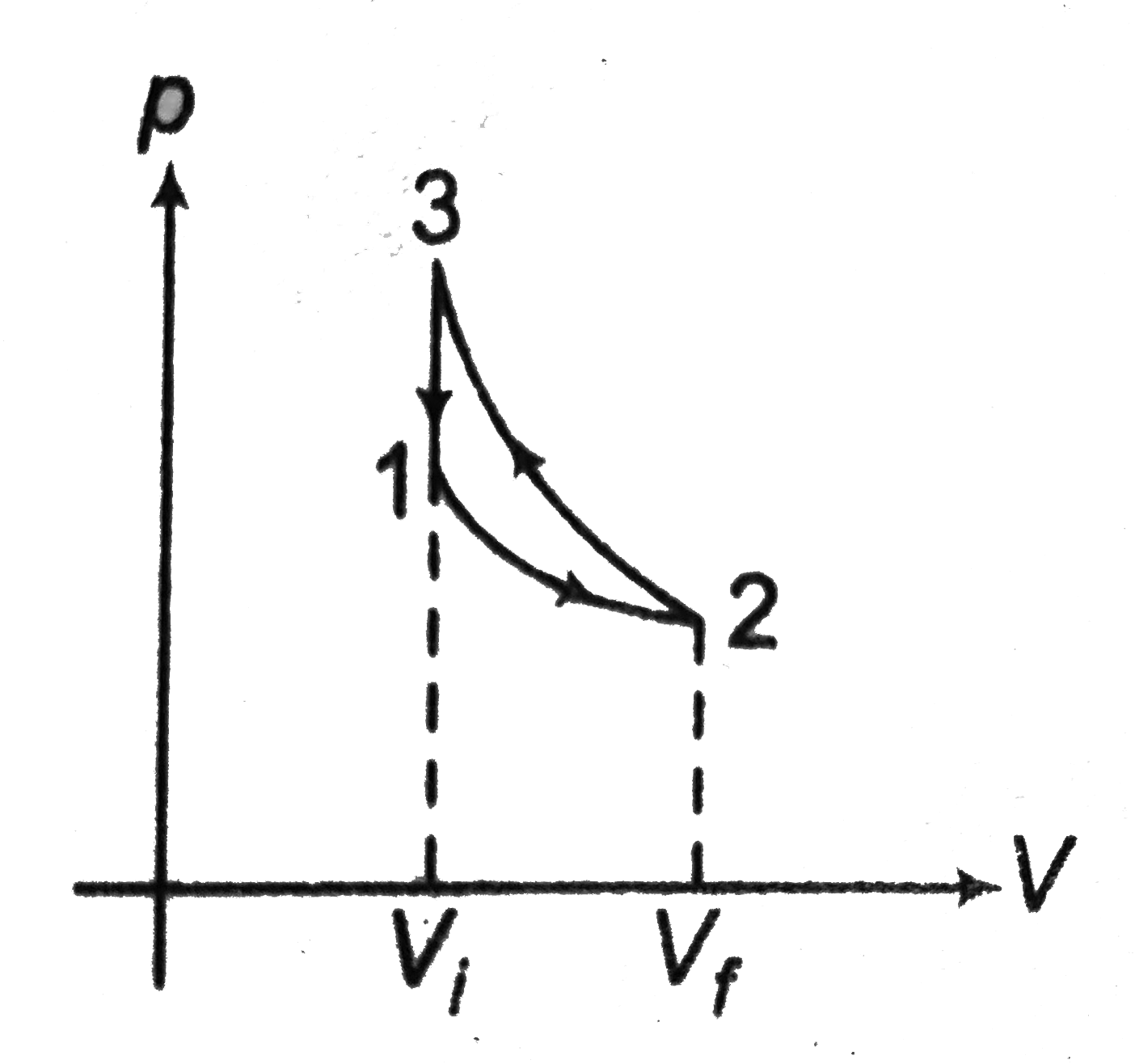
In process 3-1
V=constant
∴ T∝P
If p is decreasing, then T will also decrease.
Similar Questions
A diatomic gas initially at 18∘ is compressed adiabatically to one- eighth of its original volume. The temperature after compression will b
View SolutionA mono atomic gas initially at 27∘C is compressed adiabatically to one eighth of its original volume. The temperature after compression will be
View Solution
Knowledge Check
A diatomic gas initially at 18∘ is compressed adiabatically to one- eighth of its original volume. The temperature after compression will b
A10∘B887∘C668kD144∘An ideal gas at 27∘C is compressed adiabatically to 8/27 of its original volume. If γ=5/3, then the rise in temperature is
A275 KB375 KC475KD175KA diatomic gas initally at 18∘C is compressed adiabtically to one- eighth of its original volume. The temperature after compression will be
A144∘CB395∘CC887∘CD18∘C
A diatomic gas initially at 18∘ is compressed adiabatically to one- eighth of its original volume. The temperature after compression will b
View SolutionA monoatomic gas at pressure P1 and volume V1 is compressed adiabatically to 18th of its original volume. What is the final pressure of gas.
View SolutionOne mole of a perfect gas expands isothermally to ten times of its original volume. The change in entropy is
View SolutionA monatomic gas initially at 17∘C has suddenly compressed adiabatically to one-eighth of its original volume. The temperature after compression is
View SolutionA gas is compressed adiabatically to half its volume. By what factor does the pressure of the gas increase?
View Solution
