The energy levels of a hypothetical one electron atom are shown in the figure. (a)Find the ionization potential of this atom.
(b) Find the short wavelength limit of the series terminating at n=2
(c ) Find the excitaion potential for the state n=3.
(d) find wave number of the photon emitted for the transiton n=3 to n=1 ∝__________0eV
n=5____________−0.80eV
n=4 ______________ -1.45 eV<br>n=3____________−3.08eV
n=2_______________ -5.30 eV<br>n=1 ____________________ -15.6 eV.
The correct Answer is:A, B, C, D
(a) lonization energy = 15.6 eV
∴lonizationpotential=15.6V
(b) E∝−E2=5.3eV
:. Lambda = (12375)/(5.3) = 2335 Å
(c ) E3−E1=12.52eV
∴Excitationpotentialis12.52V.
(d) E3−E1=12.52eV
∴λ=1237512.52Å
=988.41Å
∴Waveνmber=1λ=1988.41×10−10m−1
=1.01×107m−1
Similar Questions
Calculate for a hydrogen atom the ionization potential, the first excitation potential and the wavelength of the resonance line (n'=2→n=1).
View SolutionThe energy levels of a hypotherical one electron atom are shown in figure
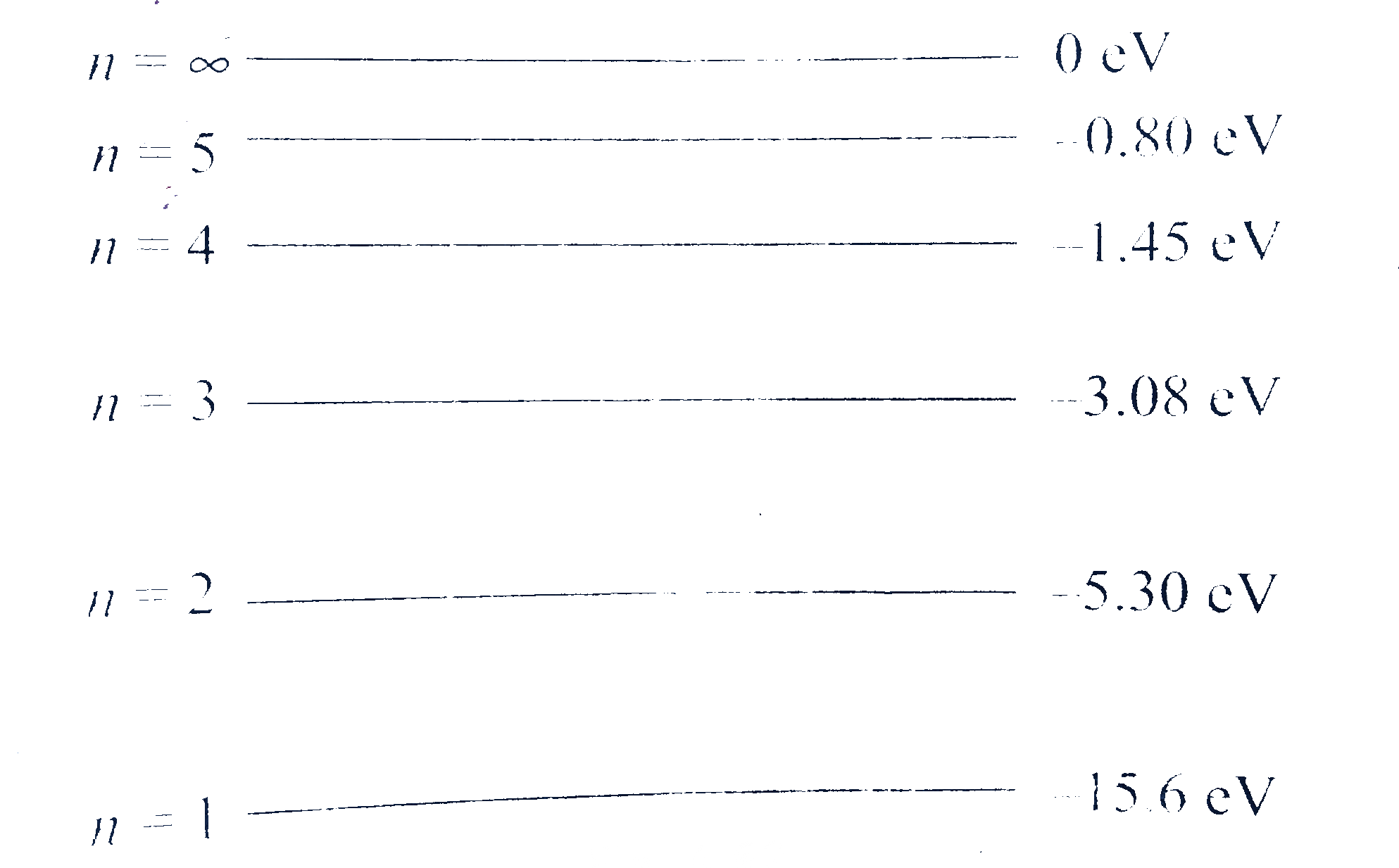
Find the wave number of the photon emitted for the transition n=3→n=1
View Solution
Knowledge Check
The energy levels of a hypotherical one electron atom are shown in figure
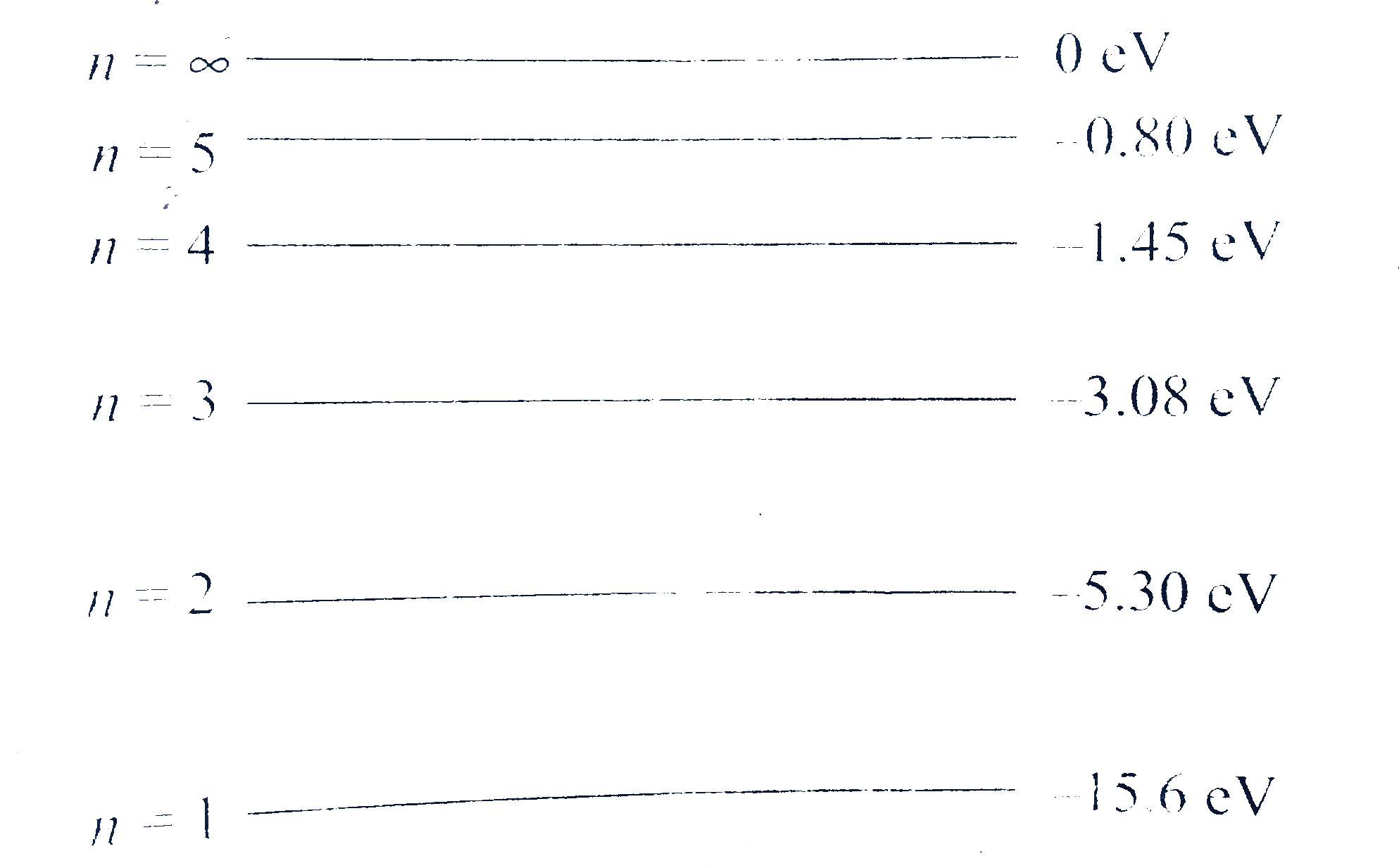
Find the ionization potential of the atom.A11.2eVB13.5eVC15.6eVD12.6eVThe energy levels of a hypotherical one electron atom are shown in figure
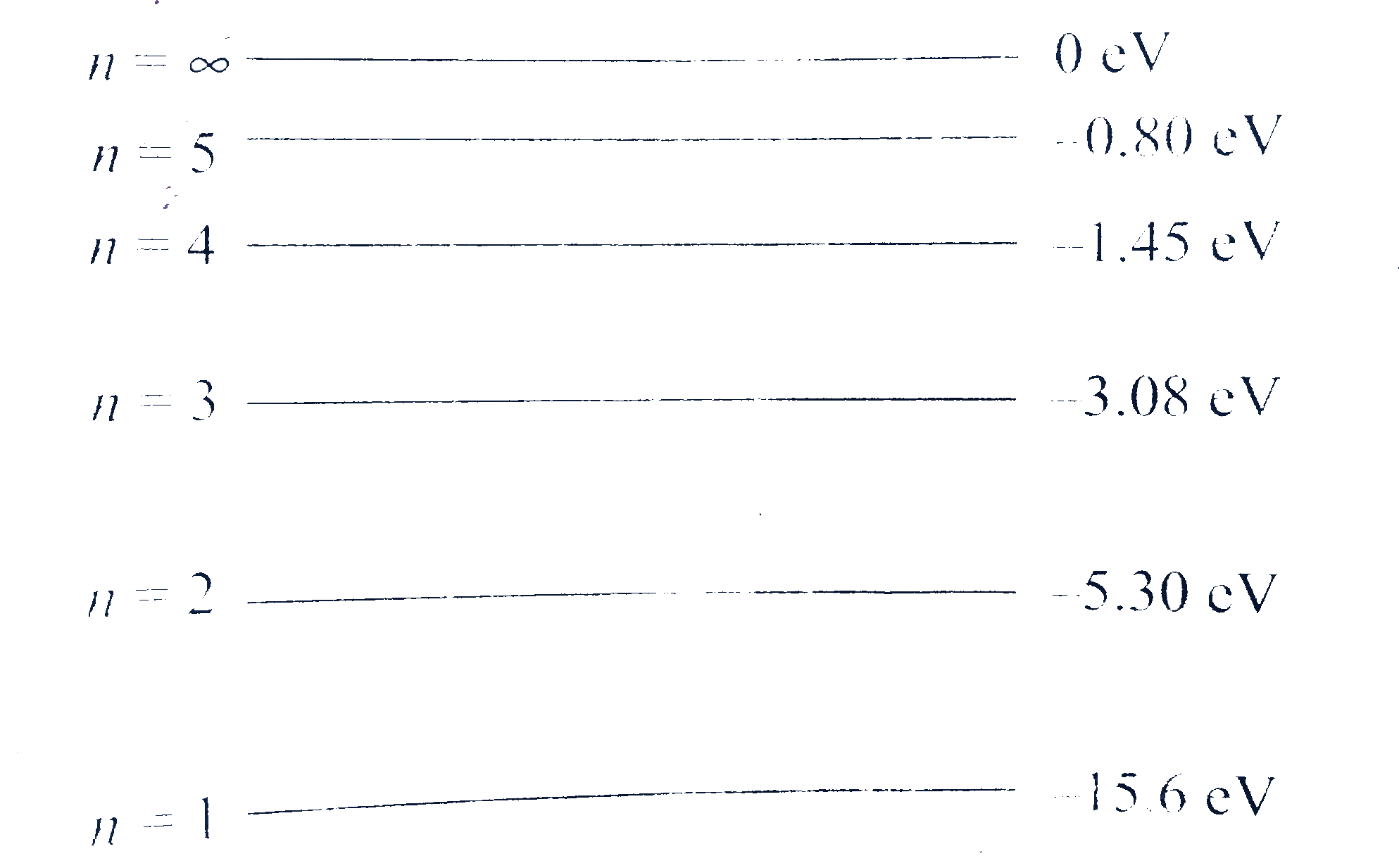
Find the excitation potential for the state n=3.A14.64eVB9.93eVC12.52eVD10.04eVThe energy levels of a hypotherical one electron atom are shown in figure
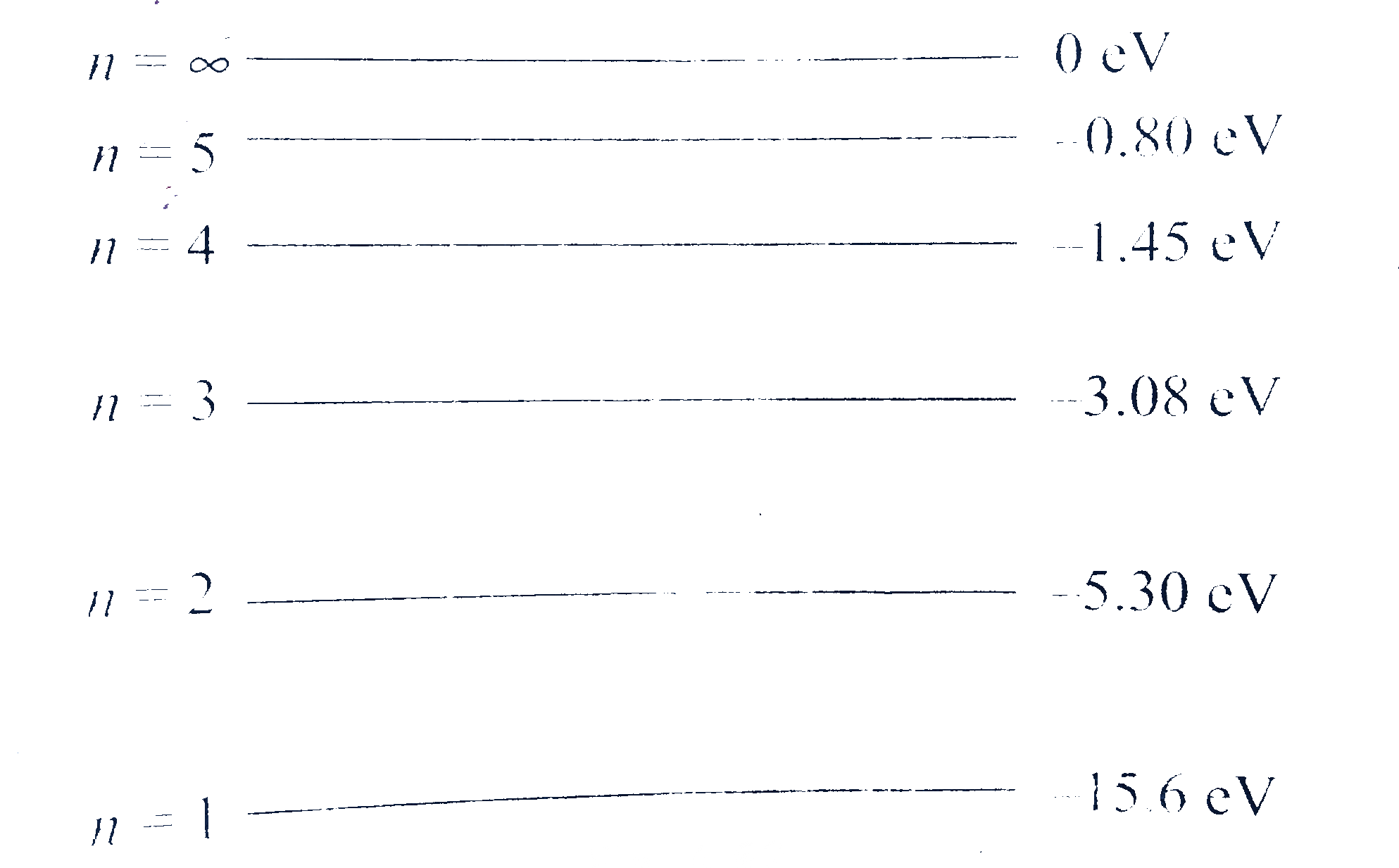
Find the short wavelength limit of the series terminating at n=2.A3256ÅB2339ÅC2509ÅD3494Å
If the energy levels of a hypothetical one electron atom are shown in the figure then find the INCORRECT option.
n=∞ _____________________________ 0eV
n=5 _____________________________ −0.80eV
n=4 _____________________________ −1.45eV
n=3 _____________________________ −3.08eV
n=2 _____________________________ −5.30eV
n=1 _____________________________ −15.6eV
View SolutionEnergy of the lowest level of hydrogen atom is −13.6eV. The energy of the photon emitted in the transition from n=3 to n=1 is
View SolutionThe energy of the ground state of hydrogen atom is −13.6 eV. The energy of the photon emitted in the transiton from n=4 to n=2 is
View SolutionThe ionization potential of a hydrogen atom is 13.6 eV. What will be the energy of the atom corresponding to n=2?
View SolutionFind the excitation energy of n = 3 level of He atom
View Solution

