Choose the correct options.
A
(a) By gamma radiations atomic number is not changed
B
(b) By gamma radiations mass number is not changed
C
(c) By the emission of one α and two β particles isotopes are produced
D
(d) By the emission of one α and four β particles isobars are produced
Video Solution
Text Solution
The correct Answer is:A, B, C
By the emission of an α-particle, atomic number decreases by 2 and by the emission of two particles atomic number increases by 2. Hence, net atomic number remains unchanged.
|
Similar Questions
Knowledge Check
Choose the correct option(s)
A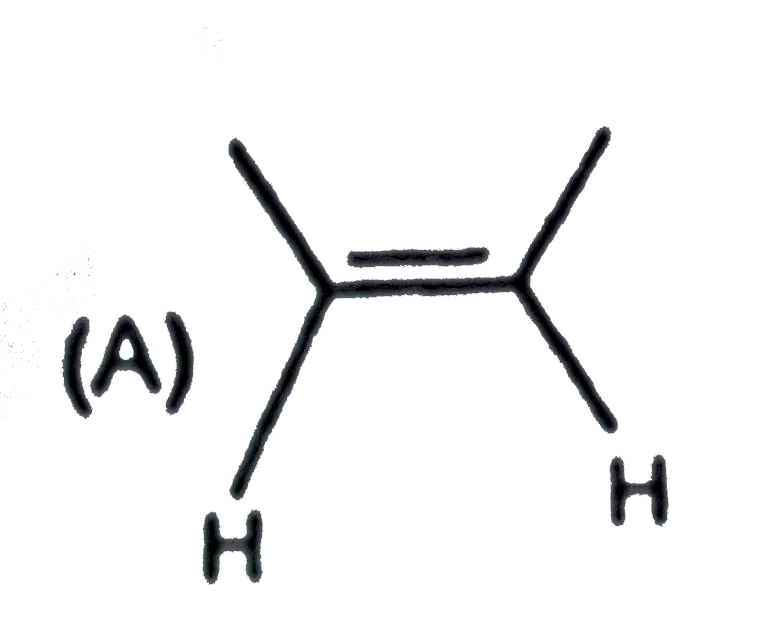 HOBr−−−→ Major products are enantiomersB
HOBr−−−→ Major products are enantiomersB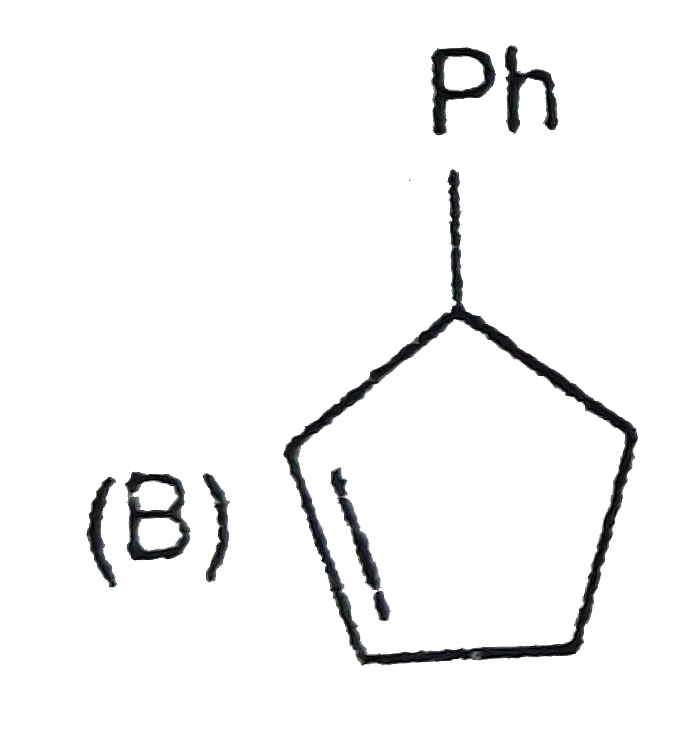 H3O⊕−−−→ Major products is optically activeCCH2=CH2OsO4/NaHSO3−−−−−−−−−→ product is CH2∣OH−CH2∣OHD
H3O⊕−−−→ Major products is optically activeCCH2=CH2OsO4/NaHSO3−−−−−−−−−→ product is CH2∣OH−CH2∣OHD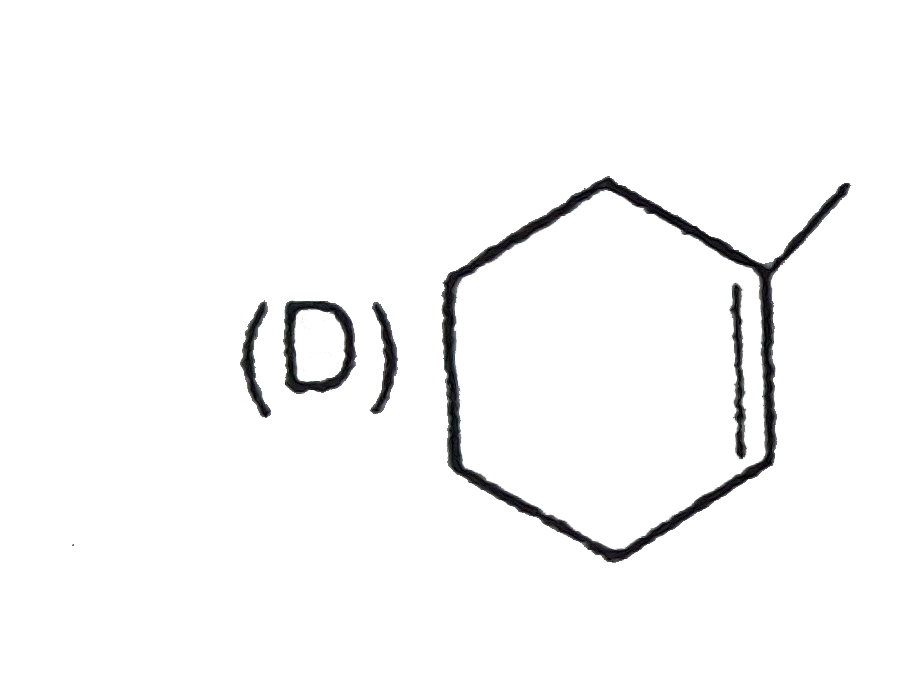 MCPBA−−−−→ products are diasteromers
MCPBA−−−−→ products are diasteromersChoose the correct option(s)
AAt low pressure (nearly 1 atm), compressibility factor for H2 gas is greater than 1 at 273 K.BCompressibility factor for a vander Waal's gas at its critical condition is less than 1CBoyle's temperature of a gas is lesser than its critical temperatureDvander Waal's constant 'a' for NH3 is greater than that of CH4Choose the correct option(s)
A HOBr−−−→ Major products are enantiomersB
HOBr−−−→ Major products are enantiomersB H3O⊕−−−→ Major products is optically activeCCH2=CH2OsO4/NaHSO3−−−−−−−−−→ product is CH2∣OH−CH2∣OHD
H3O⊕−−−→ Major products is optically activeCCH2=CH2OsO4/NaHSO3−−−−−−−−−→ product is CH2∣OH−CH2∣OHD MCPBA−−−−→ products are diasteromers
MCPBA−−−−→ products are diasteromers