Shape of O2F2 is similar to that of
A
C2F2
B
H2O2
C
H2F2
D
C2H2
Video Solution
Text Solution
The correct Answer is:B
O2F2 and H2O2, both have open book tpe structure.
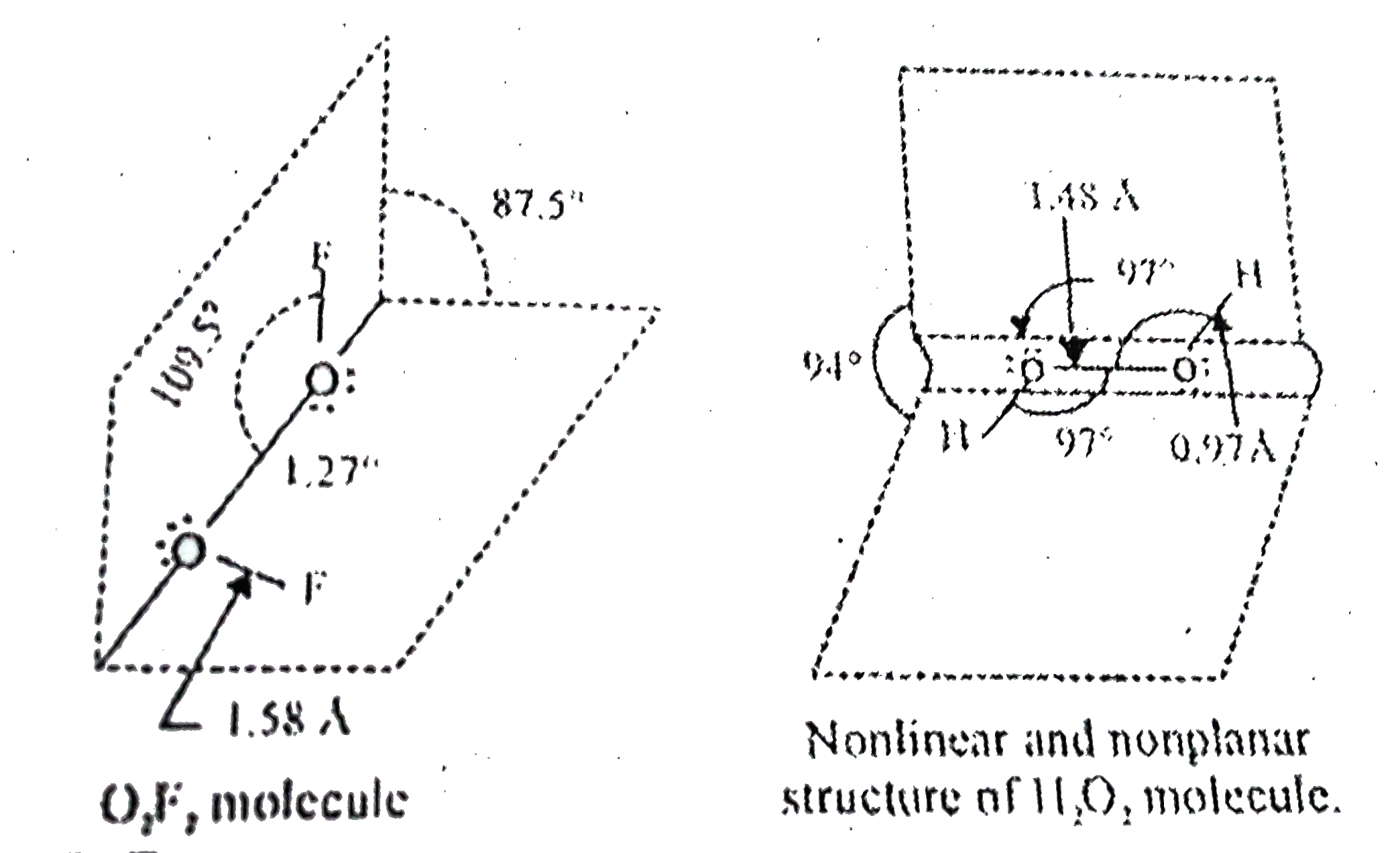
in O2F2 one O-O and two O-F bonds are lying in different plane,i.e.this molecule like H2O2 has non-linear and non-planner structure.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Shape of O2F2 is similar to that of
AC2F2BH2OCH2F2DC2H2The shape of NH3 is very similar to that of :
ABH3BCH−3CCH+3DSO2−4Shape of NH3 is very similar to :
ACH4BCH−3CBH3DCH+3
Assertion :H0O2is non − ionic compound.
Reason : The O−O bond length in H2O2 is shorter than that of O2F2.
View SolutionThe shape of H2O2 is dissimilar to:
View SolutionShape of H2O2 molecule is
View SolutionThe shape of methyl carbanion is similar to that of -
View SolutionAssertion. The O-O bond length in H2O2 is shorter than that of O2F2.
Reason. H2O2 is an ionic compound.
View Solution
