During estimation of nickel , we prepare nickel dimethylglyoxime, a scarlet red solid. This compound is _______.
ionic
covalent
metallic
non-ionic complex.
The correct Answer is:D
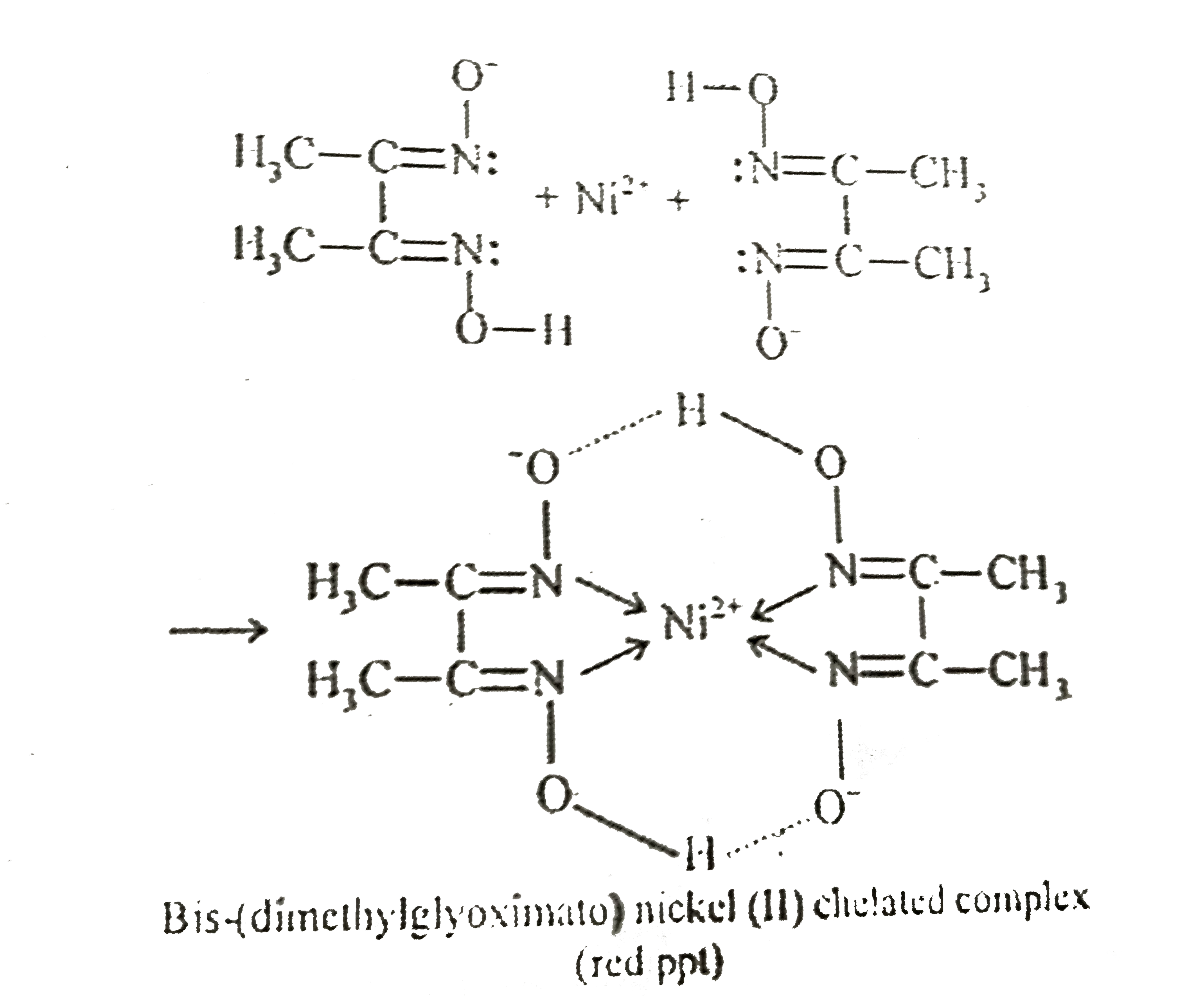
This planar complex is very poorly soluble and is used for the gravimetric determination of nickel e.g. ores.
Similar Questions
Can we estimate oxygen in a compound ?
View SolutionWhich of the following statements is correct?
(a). Nickel is a silver white metal.
(b). Nickel dimethylglyoxime is square planer.
(c). Among complexes of cobalt the most stable are those of Co2+,
(d). Among simple compounds of cobalt the most stable are those of CO2+.
View Solution
Knowledge Check
During estimation of nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.5 g of the compound in Kjeldahl’s estimation of nitrogen, neutralized 10 mL of 1 M H2SO4. Find out the percentage of nitrogen in the compound.
A0.56B0.46C0.6D0.36In the electroplating of iron by nickel, nickel sulphate solution is taken as an electrolyte. What is the acid that is added to the electrolyte during the process ?
AHClBHCNCHNO3DH2SO4
During estimation of nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.5 g of the compound in Kjeldahl’s estimation of nitrogen, neutralized 10 mL of 1 M H2SO4. Find out the percentage of nitrogen in the compound.
View SolutionDuring estimation of nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.5 g of the compound in Kjeldahl’s estimation of nitrogen, neutralized 10 mL of 1 M H2SO4. Find out the percentage of nitrogen in the compound.
View SolutionHow is nickel detected and estimated in a salt ? Give the reaction involved.
View SolutionFind out the number of hydrogen bonds present in the structure of the nickel dimethylglyoxime complex .
View SolutionWhen diemethyl glyoxime is added to the aqueous solution of nickel (II) chloride in presence of dilute ammonia solution, a bright red coloured precipitate is obtained .
(a) Draw the structure of bright red substance .
(b) Write the oxidation state of nickel in the substance and hybridisation.
(c ) State whether the substance is paramagnetic or diamagnetic.
View SolutionThe oxygen is estimated in the organic compound by
View Solution
