Assertion : Tyrosine behave as a acidic at pH =7
Reason: pK8 of phenol is mole than 7.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both asertion and reason are false.
The correct Answer is:A
Topper's Solved these Questions
Similar Questions
Sodium metal crystallises in a body centred cubic lattice with edge length of the unit cell equal to 4.29Å The radius of the sodium atom is approximately
View SolutionComplete the following reaction
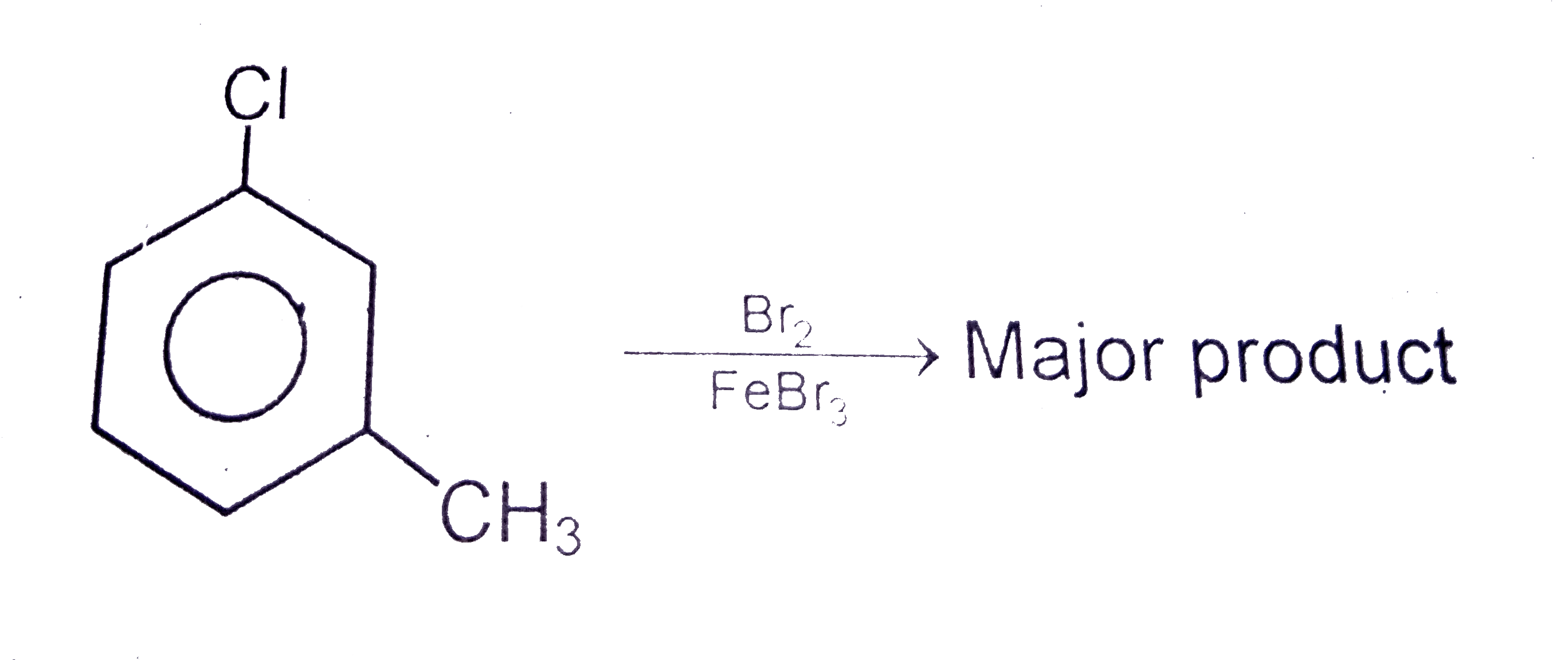
View SolutionAssertion (A): ph of acidic solution is always below 7 at 25^(@)C . Reason (R) : At 25^(@)C , the pH of 10^(-8)M HCI is 8 .
View SolutionAssertion : The pH of a basic buffer mixture is given by: pH=pK_(a)+log(([Base])/([conjugate acid])) Reason : The pH of an acidic buffer mixture is given by: pH=pK_(a)+log(([conjugate acid])/([Acid]))
View SolutionAssertion: The pKa of acetic acid is lower than that of phenol.
Reason : Phenoxide ion is more resonance sabilised.
View SolutionAssertion: The pK_(a) of acetic acid is lower than that of phenol Reason: Phenoxide ion is more resonance stabilised.
View SolutionAssertion: The pKa of acetic acid is lower than that of phenol.
Reason : Phenoxide ion is more resonance sabilised.
View SolutionAssertion : pH of the buffer solution is not affected by dilution.
Reason : pH=pKa+log[Conjugate acid][Base]
View SolutionAssertion: The pKa of acetic acid is lower than that of phenol.
Reason : Phenoxide ion is more resonance sabilised.
View Solutionअम्ल का pH मान 7 से अधिक होता है |
View SolutionAssertion : pK_(a) value of acetic acid is lower than that of phenol Reason : Phenoxide ion is more resonance stabilised than acetate ion
View SolutionpH less than 7 : acid :: pH greater than 7 : ______
View Solutionकथन :- उदासीन pH पर टायरोसीन अम्ल की तरह व्यवहार करता है।
कारण :- फिनॉल के pKa का मान 7 से अधिक होता है। |
View SolutionAssertion : pH of the buffer solution is not affected by dilution. Reason : pH=pK_(a)+"log"(["Conjugate base"])/(["Acid"])
View SolutionAssertion (A): pH of acidic solution is always below 7 at 25∘C.
Reason (R) : At 25∘C, the pH of 10−8MHCI is 8.
View Solution

