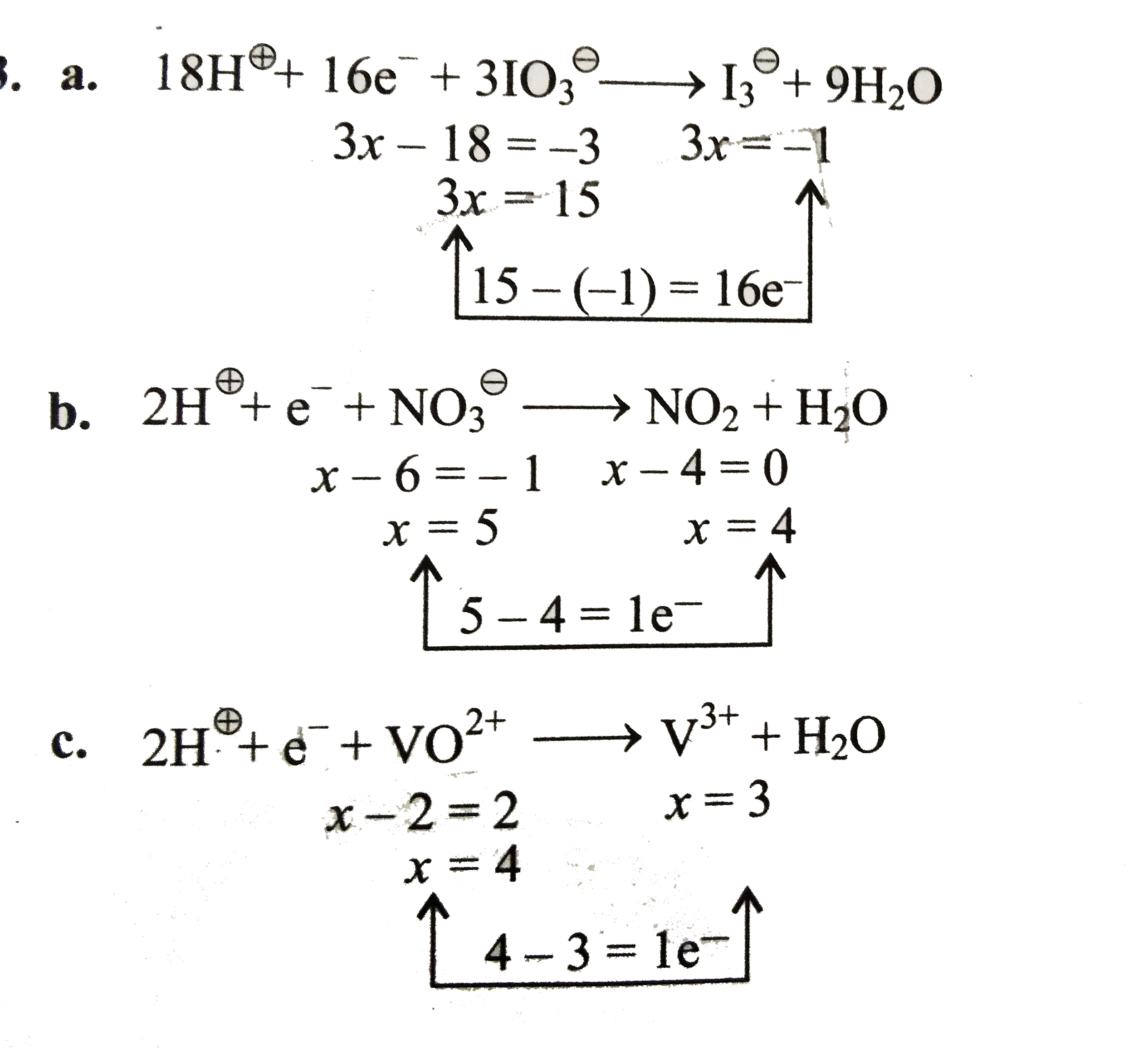
Balance the following half-reactions in acidic medium:
(a) IOӨ3(aq)→IӨ3(aq)
(b) NOӨ3(aq)→NO2(g)
( c) VO2−(aq)→B3+(aq)
The correct Answer is:A
Similar Questions
4M(s)+overset(Ө)(C)N(aq.)+2H_(2)O(aq.)+O_(2)(g)
" " to 4[M(CN)_(2)]^(Ө)(aq.)+4overset(Ө)(O)H(aq.)
View SolutionSuggest a scheme of classification of the following redox reactions
(a) N2(g)+O2(g)→2NO(g)
(b) 2Pb(NO3)2(s)→2PbO(s)+4NO2(g)+O2(g)
(c) NaH(s)+H2O(l)→NaOH(aq)+H2(g)
(d) 2NO2(g)+2OH−(aq)→NO−2(aq)+NO−3(aq)+H2O(l)
View SolutionClassify the following redox reactions:
a. N2(g)+O2(g)→2NO(g)
b. 2Pb(NO)3(s)→2PbO(s)+2NO2(g)+12O2(g)
c. NaH(s)H2O(l)→NaOH(aq)+H2(g)
d. 2NO_(2)(g)+2overset(ө)OH(aq)rarrNO_(2)^(ө)(aq)+NO_(3)^(ө)(aq)+H_(2)O(l)
View SolutionBalance the conversions : I^(-)(aq) overset(H^(+))rarr I_(2)(g) and Fe^(2+)(aq) overset(H^(+))rarr Fe^(3+)(aq)
View SolutionIdentify the oxidant and reductant in the following reactions: a. 10H^(o+)(aq)+4Zn(s)+NO_(3)^(ө)(aq)rarr4Zn^(2+)(aq)+NH_(4)^(o+)(aq)+3H_(2)O(l) b. I_(2)(g)+H_(2)S(g)rarr2Hl(g)+S(s)
View SolutionBalance the following half-reactions in acidic medium: (a) IO_(3)^(Ө)(aq) rarr I_(3)^(Ө)(aq)
View SolutionWrite balanced ionoic half equation (oxidation and reduction) for each of the following reactions:
(a) Mn3+(aq)→MnO2(s)+Mn2+(aq)
(b) Mn(s)+NOӨ3(aq)→Mn2+(aq)+NO2(g)
(c ) H2O2(aq)+Fe2+(aq)→Fe3+(aq)+H2O(l)
(d) Te(s)+NOӨ3(aq)→TeO2(s)+NO(g)
View SolutionBalance the following half reactions in basis medium: (a) CrO_(4)^(2-)(aq)rarrCr(OH)_(4)^(Ө)(aq) (b) CIO^(Ө)(aq) rarr Cl^(Ө)(aq) (c ) Bi^(3+)(aq) rarr BiO_(3)^(Ө)(aq)
View Solution