The oxidation number of Cr is +6 in
A
FeCr2O4
B
KCrO3Cl
C
CrO5
D
[Cr(OH)_(4)]^(ө)
Video Solution
Text Solution
The correct Answer is:B, C
Fe2+(Cr2O4)2−:Oxidation state ofCr=+3
b. K⊕[CrO3Cl]ө:Oxidation state ofCr=+6
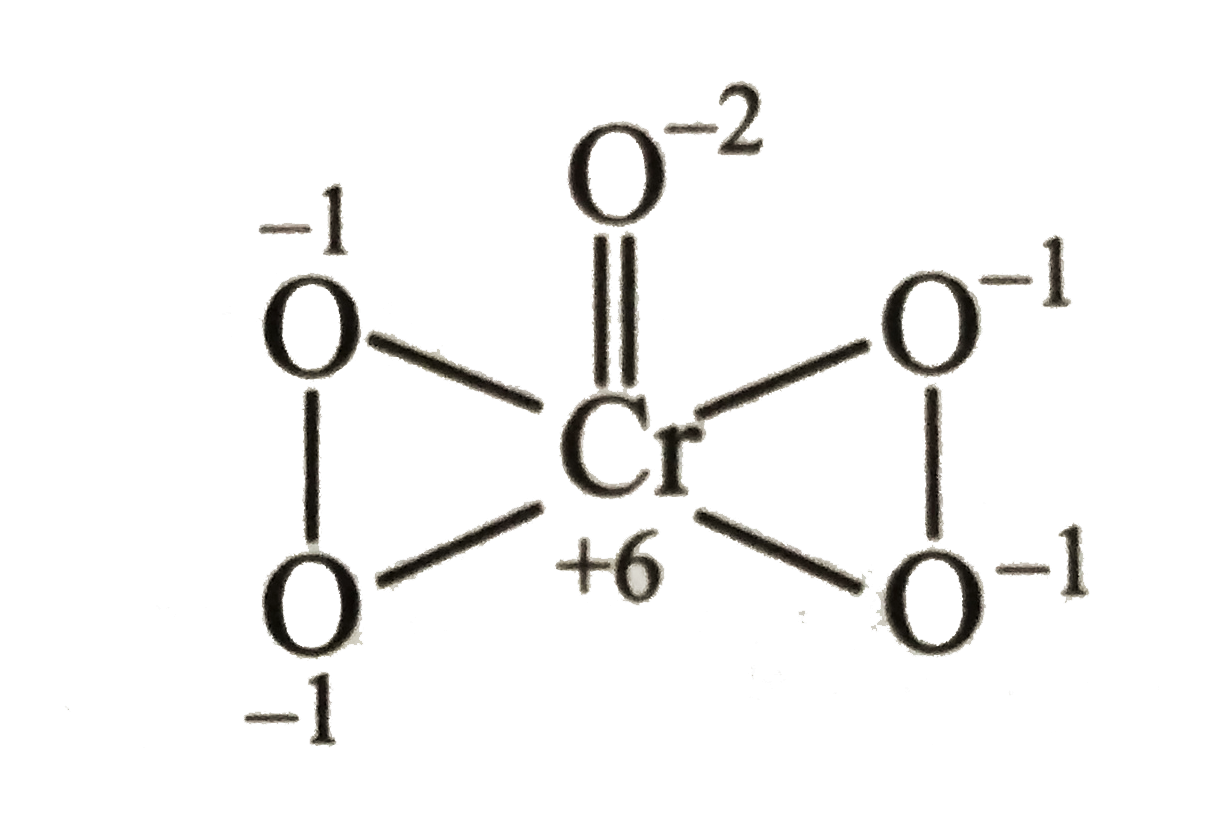
Oxidation state of Cr=+6. ( Two peroxo linkage)
d. [Cr(OH)4]ө:Oxidation state ofCr=+3
|
Similar Questions
Knowledge Check
In the complex ion, [Cr(C2O4)2(H2O)2]− the oxidation number of Cr is
A+1B+2C+3D−2The oxidation number of Cr in Cr(CO6) is :
A0B2C−2D+6In which of the following compounds of Cr, the oxidation number Cr is not +6 :-
ACrO3BCrO2Cl2CCr2O3DK2Cr2O7
The oxidation number of Cr in the product of alkaline oxidative fusion of FeCr2O4 is
View SolutionRCH2OH+CrO3aq.H2SO4−−−−−→Z+CrX+
The oxidation number 'x' of Cr will be
View SolutionSTATEMENT-1: In CrO5 oxidation number of Cr is +6.
STATEMENT-2 : CrO5 has butterfly structure in which peroxide peroxide bonds are present.
View SolutionThe oxidation number of Cr in [Cr(NH3)6]Cl3 is
View SolutionThe oxidation number of Cr in [Cr(NH3)6]Cl3 is
View Solution

