The oxidation state of chrominium in the final product formed in the reaction between KI and acidified potassium dichromate soluttion is
A
+4
B
+6
C
+2
D
+3
Video Solution
Text Solution
The correct Answer is:D
6e−+Cr2O2−7→2Cr3+
2Iө→I2+2e−
Oxidation state of Cr=+3
|
Similar Questions
Knowledge Check
The oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is :
A+3B+2C+6D+4The oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is :
A+3B+2C+6D+4The oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is :
A+3B+2C+6D+4
The oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is :
View SolutionThe oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is:
View SolutionThe final product (Y) formed in the reaction
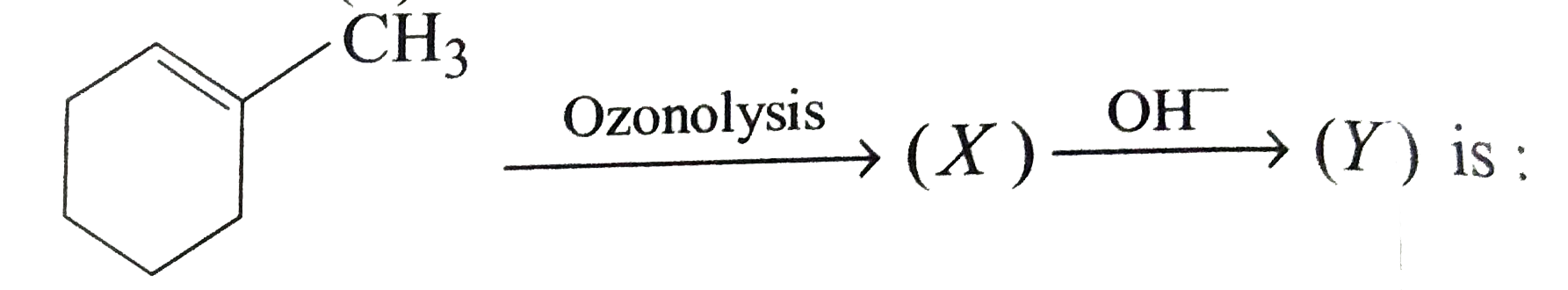
View SolutionThe final product formed in this reaction is :

View SolutionThe oxidation state of chrominium in Cr(CO)6 is
View Solution

