20 " mL of " a gasous bydrocarbon (A) was exploded with excess of O2 in an eudiometer tube. On cooling, the volume was reduced by 50 mL. On further treatment with KOH, there was further contraction of 40 mL. The molecular formula of the hydrocarbon A is
(a). C2H6
(b). C2H4
C3H6
(d). C3H8
CxHy(g)+(x+y4)O2(g)→xCO2(g)+y2H2O(l)
CO2 is absorbed in KOH.
∴20x=40⇒x=2
20mL+20(2+y4)=40+50
20+40+20y4=90
5y=90−60=30
y=6
Formula of the compound is C2H6.
Similar Questions
16 mL of a hydrocarbon gas was exploded with excess of oxygen. On cooling, the volume of the resulting gaseous mixture was reduced by 48 mL. When KOH was added, there was a further decrease of 48 mL in the volume. Find the molecular formula of the compound.
View Solution16 mL of hydrocarbon gas was exploded with excess of oxygen on cooling the volume of resulting gaseous mixture was reduced by 48 mL When KOH added there was further decreases of 48 mL in volume. Find the molecules formula.
View Solution
Knowledge Check
Twenty millilitres of a gaseous hydrocarbon (A) was exploded with excess of oxygen in eudiometer tube. On cooling, the volume was reduced by 50ml. On further treatement with KOH solution, there was a further contraction of 40 ml.
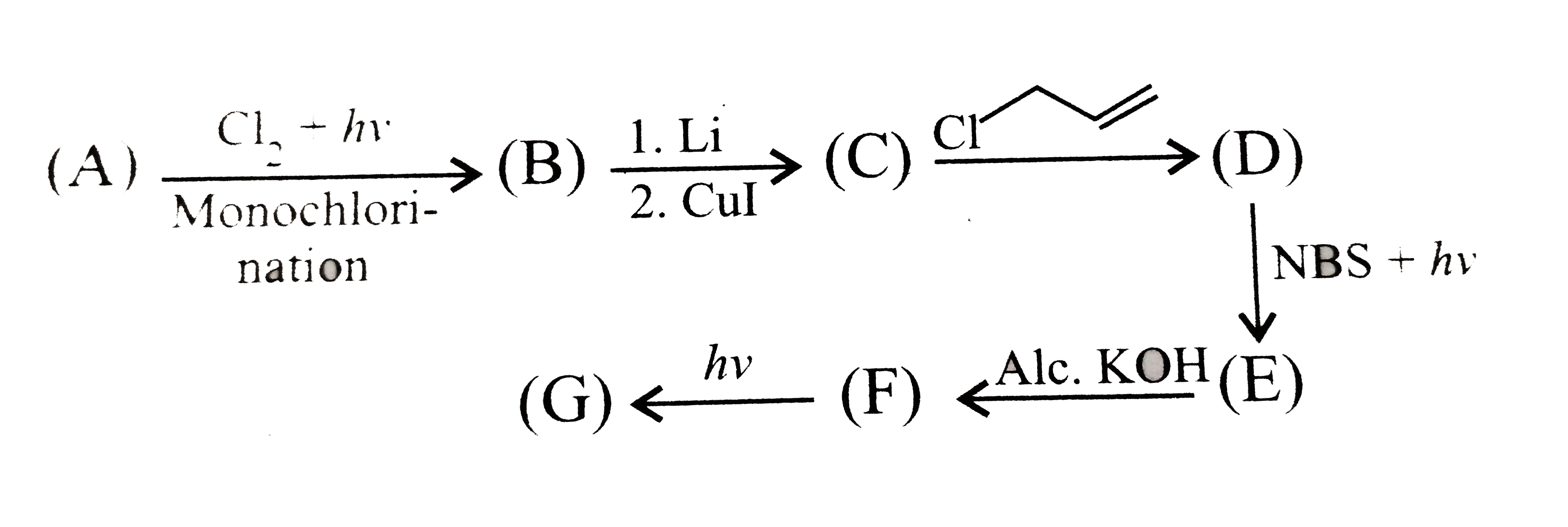
The molecular formula of the hydrocarbon (A) is:AC2H6BC2H4CC3H6DC3H8Twenty millilitres of a gaseous hydrocarbon (A) was exploded with excess of oxygen in eudiometer tube. On cooling, the volume was reduced by 50ml. On further treatement with KOH solution, there was a further contraction of 40 ml.
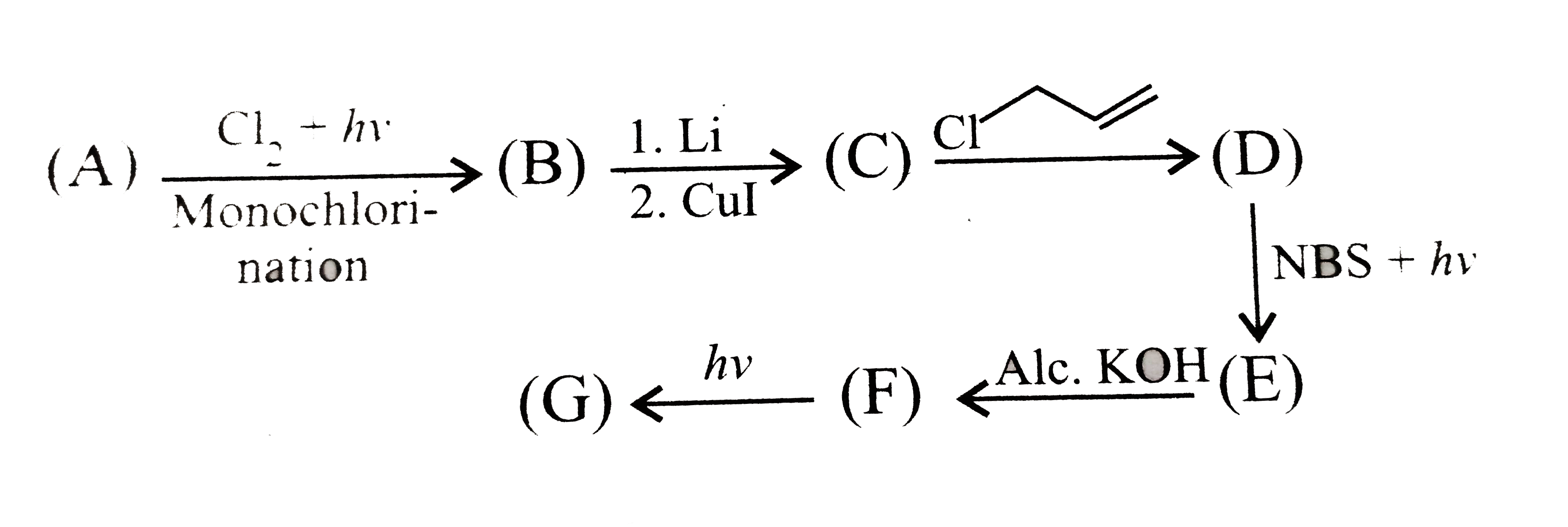
Compound (C) is:A(C2H5)2CuLiB(C3H7)2CuLiC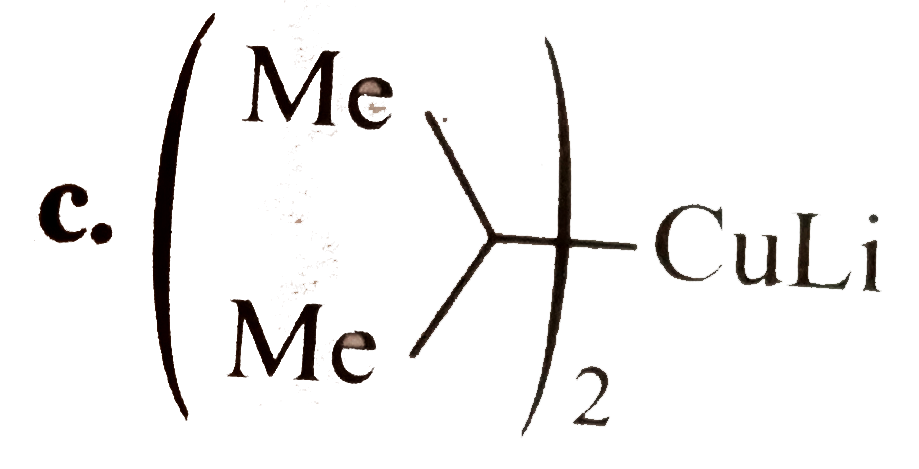 D
D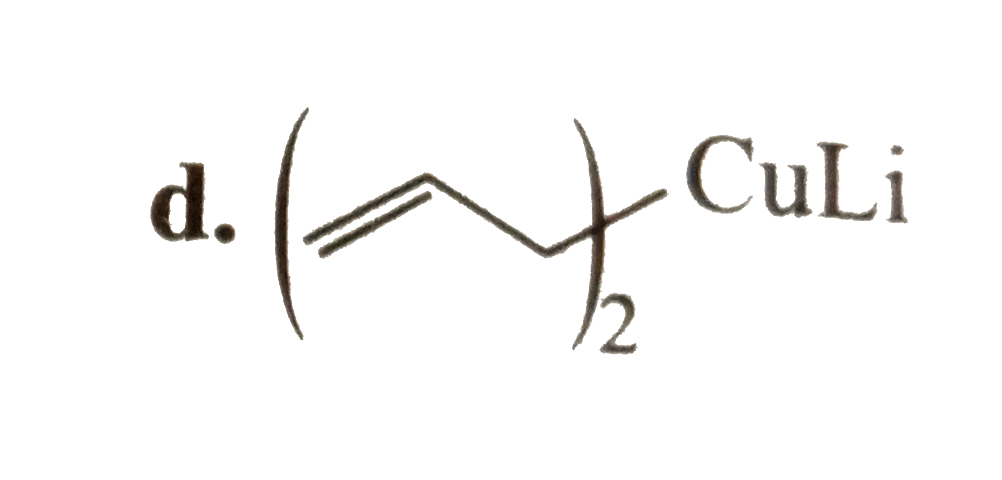
Twenty millilitres of a gaseous hydrocarbon (A) was exploded with excess of oxygen in eudiometer tube. On cooling, the volume was reduced by 50ml. On further treatement with KOH solution, there was a further contraction of 40 ml.
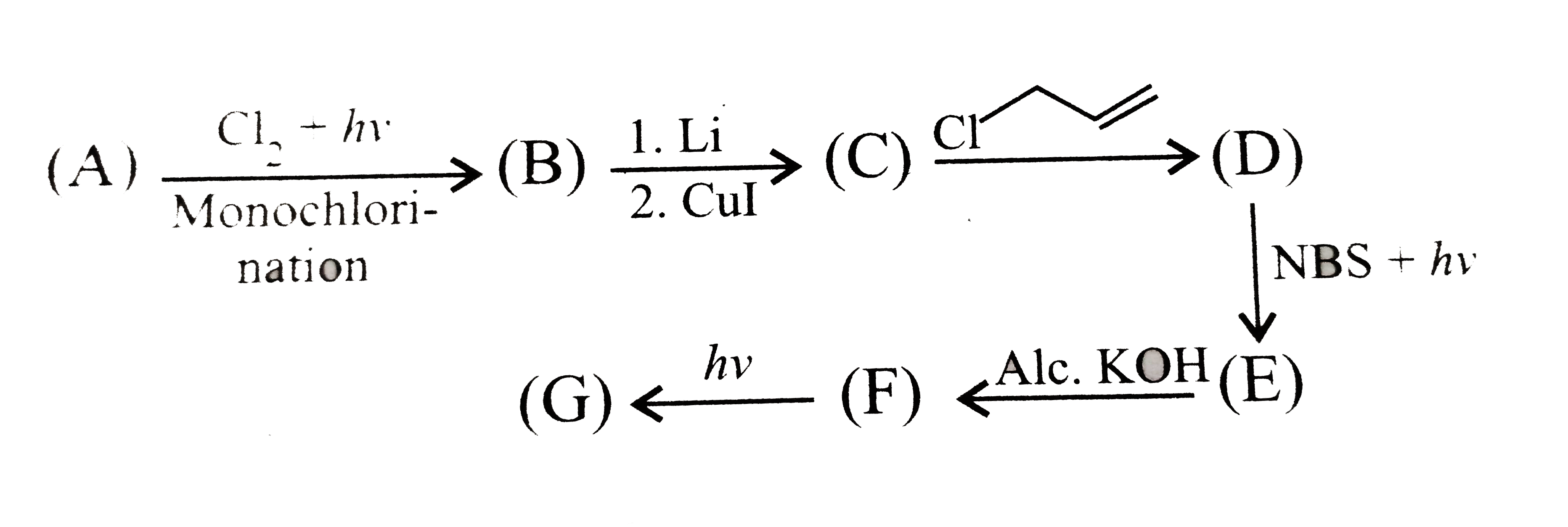
Compound (D) is:A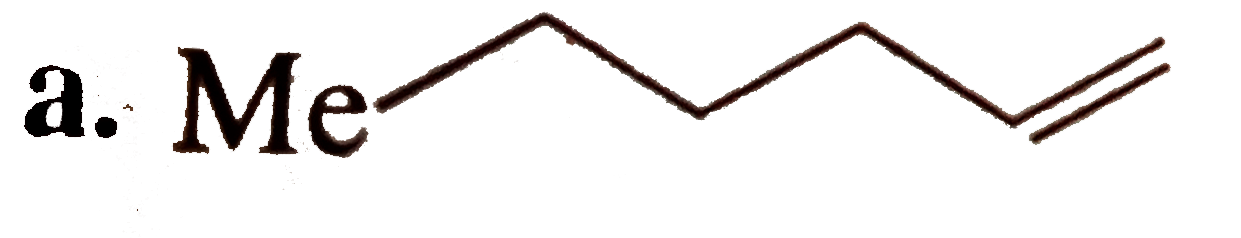 B
B C
C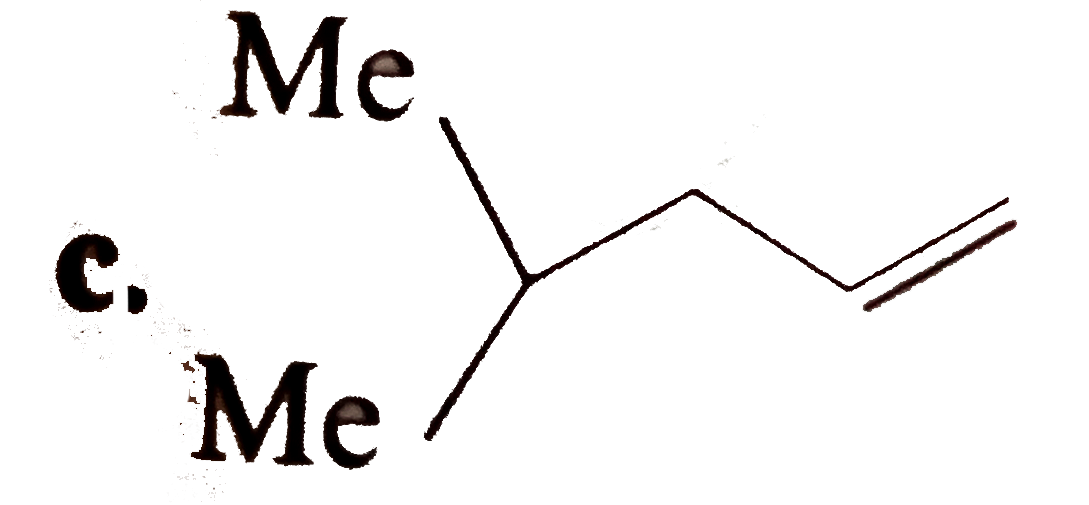 D
D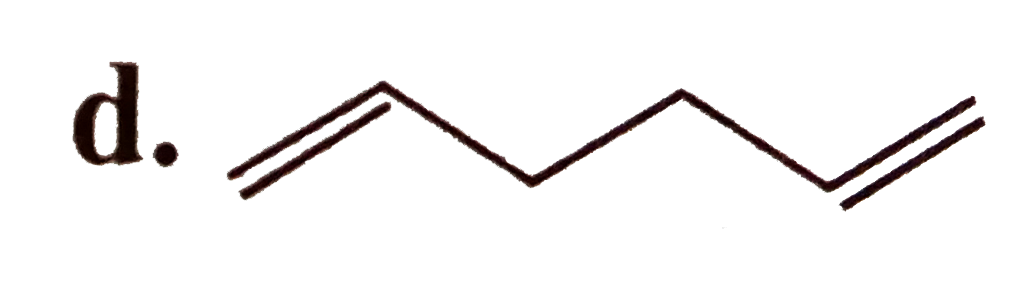
8.4 " mL of " gaseous hydrocarbon A was burnt with 50 " mL of " O2 in a eudiometer tube. The volume of the products after cooling to room temperature was 37.4 mL. When reacted with NaOH, the volume contracted to 3.8 mL. What is the molecular formula of A.
View SolutionTwenty millilitres of a gaseous hydrocarbon (A) was exploded with excess of oxygen in eudiometer tube. On cooling, the volume was reduced by 50ml. On further treatement with KOH solution, there was a further contraction of 40 ml.
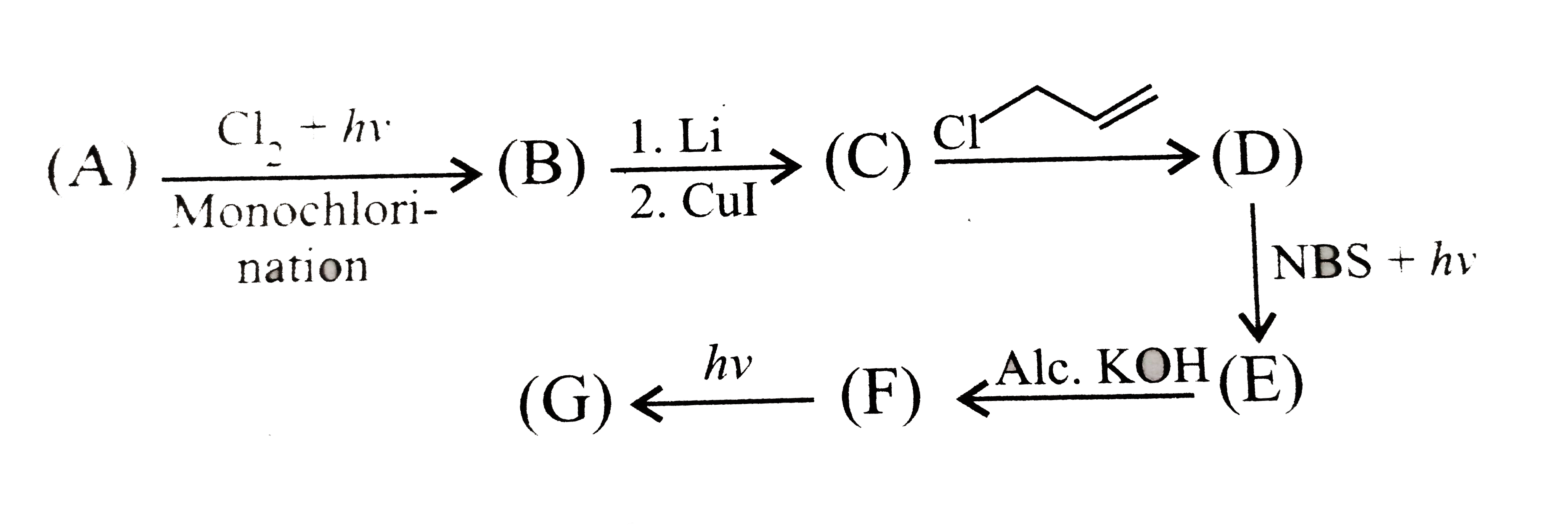
Compound (E) is:
View SolutionTwenty millilitres of a gaseous hydrocarbon (A) was exploded with excess of oxygen in eudiometer tube. On cooling, the volume was reduced by 50ml. On further treatement with KOH solution, there was a further contraction of 40 ml.
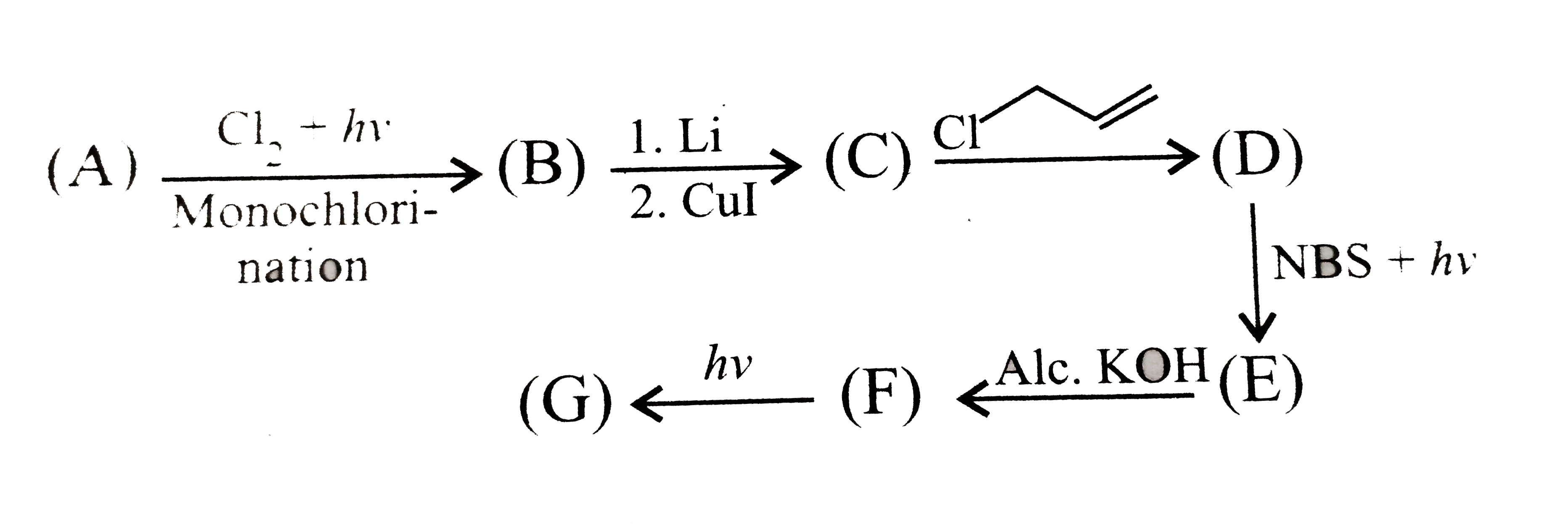
Compound (G) is:
View SolutionTwenty millilitres of a gaseous hydrocarbon (A) was exploded with excess of oxygen in eudiometer tube. On cooling, the volume was reduced by 50ml. On further treatement with KOH solution, there was a further contraction of 40 ml.
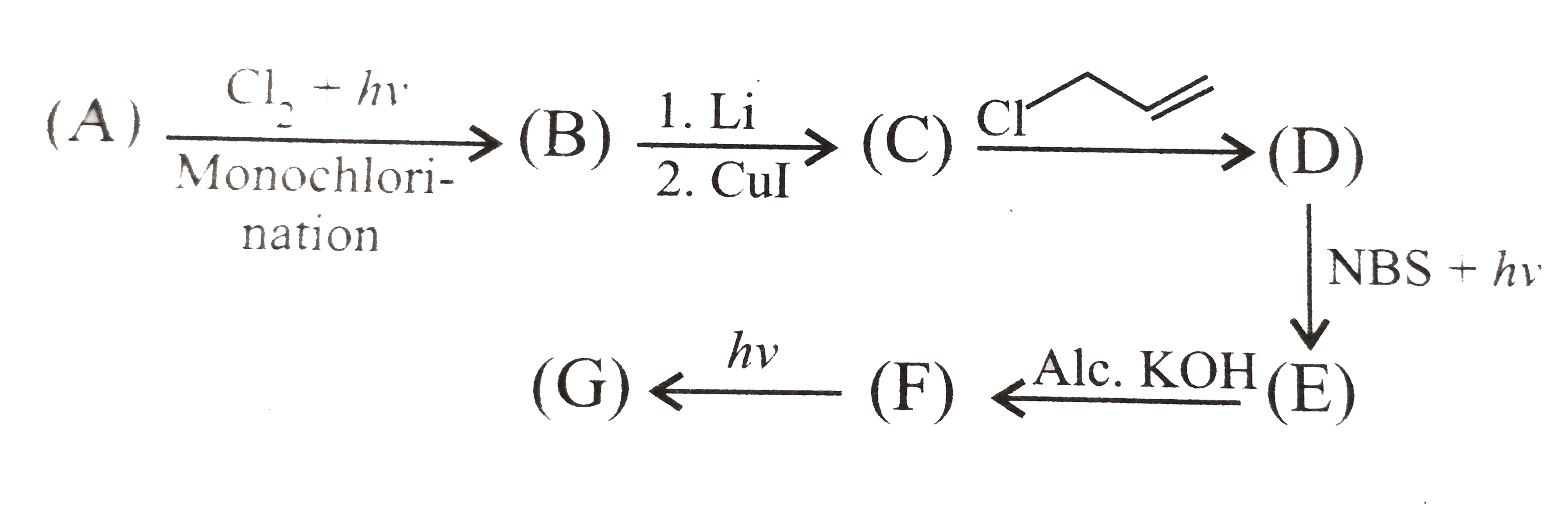
Compound (F) is:
View Solution8.4 ml of a gaseous hydrocarbon was burnt with 50 ml of O2 in a eudiometer tube. The volume of the products formed after cooling to room temperature was 37.4 ml, when reacted with NaOH, the volume contracted to 3.8 ml. The molecular formula of the hydrocarbon is
View Solution
