The correct set of quantum number for the unpaired electron of chlorine atom is
nlm1 " " nlm1
A
210
B
211
C
11
D
300
Video Solution
Text Solution
The correct Answer is:C
|
Answer
Step by step video, text & image solution for The correct set of quantum number for the unpaired electron of chlorine atom is {:(n,l,m_(1)):} " " {:(n,l,m_(1)):} by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The correct set of quantum numbers for the unpaired electron of chlorine atom is
Anlm210Bmlm211Cnlm311Dnlm300The correct set of quantum numbers for the unpaired electron of chlorine atom is
An=2,l=1,m=0Bn=2,l=1,m=1Cn=3,l=1,m=1Dn=3,l=0,m=0The correct set of quantum number for the unpaired electron of chlirine atom is
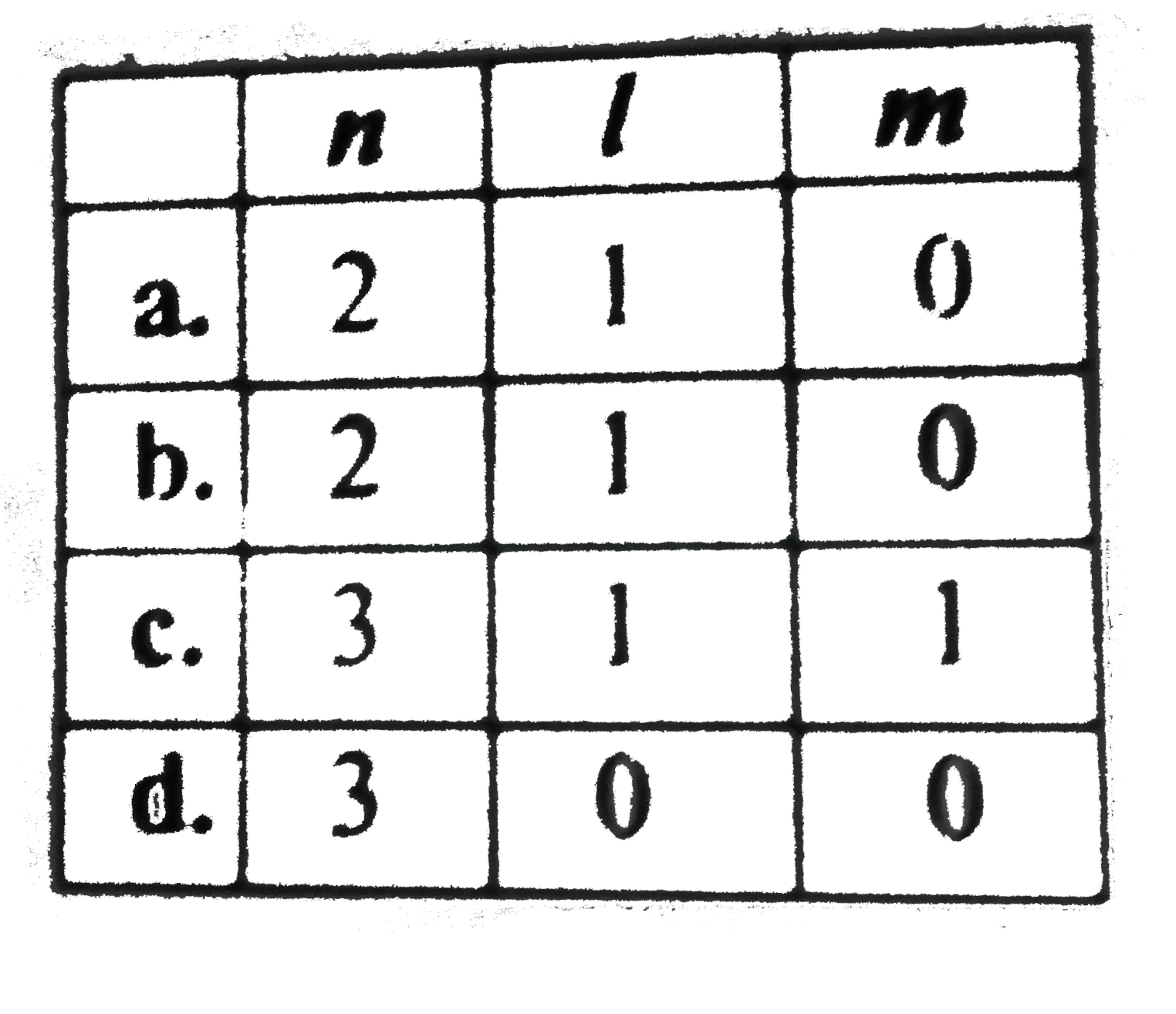 An=2,l=1,m=0Bn=2,l=1,m=0Cn=3,l=1,m=1Dn=3,l=0,m=0
An=2,l=1,m=0Bn=2,l=1,m=0Cn=3,l=1,m=1Dn=3,l=0,m=0
The correct set of quantum number for the unpaired electron of a chlorine atom is
View SolutionThe correct set of quantum numbers for the unpaired electron of chlorine atom is :
View SolutionThe correct set of n, l, m, quantum numbers for the unpaired electron of chlorine atom is:
View SolutionThe correct set of quantum numbers for the unpaired electron of Cu atom is :
View SolutionThe correct set of quantum numbers (n, I and m respectively) for the unpaired electron of chlorine atom is
View Solution
