The total allowed values of l for an given value of n are equal to …………..
n
Similar Questions
The total allowed values of m for an given value of l are equal to …………..
View SolutionThe magnetic quantum number is denoted by letter m, and for a given value of I, it can have all the values ranging from -I to +I including zero. For a given I,m has 2l + 1 values. For example, if l = 2, m can have values, i.e., m = -2, -1,0, +1, +2. This implies that there are five different orientations of the d-subshell. In other words, d- subshell has five d-orbitals Which of the following is correct for 2p-orbitals? a)n = 1, l = 2 b)n = 1, l = 0 c)n = 2,l = 0 d)n = 2,l = 1
View Solution
Knowledge Check
The maxium value of l for n =4 is
A2B4C3D5In given circle value of θ is equal to
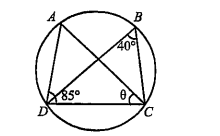 A35∘B45∘C55∘D65∘
A35∘B45∘C55∘D65∘The total number of m values for n=4 is
A8B16C12D20
What are the possible values of l for an electron with n=3 ?
View SolutionStatement -1:- For n=2 the values of l may be 0,1 and m may be 0,±1.
Statement -2 :- for each value of n, there are 0 to (n-1) possible values of l for each value of la there are 0 to ± l values of m.
View SolutionIn given parallelogram ABCD values of x and y are equal to
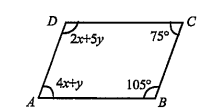
View SolutionThe possible values of l depend on the value of the principal quantum number n. For a given value of n,l has possible intergal values from
View Solutionloganlogabn=1+logab
View Solution
