The pressure-volume of varies thermodynamic process is shown in graphs:
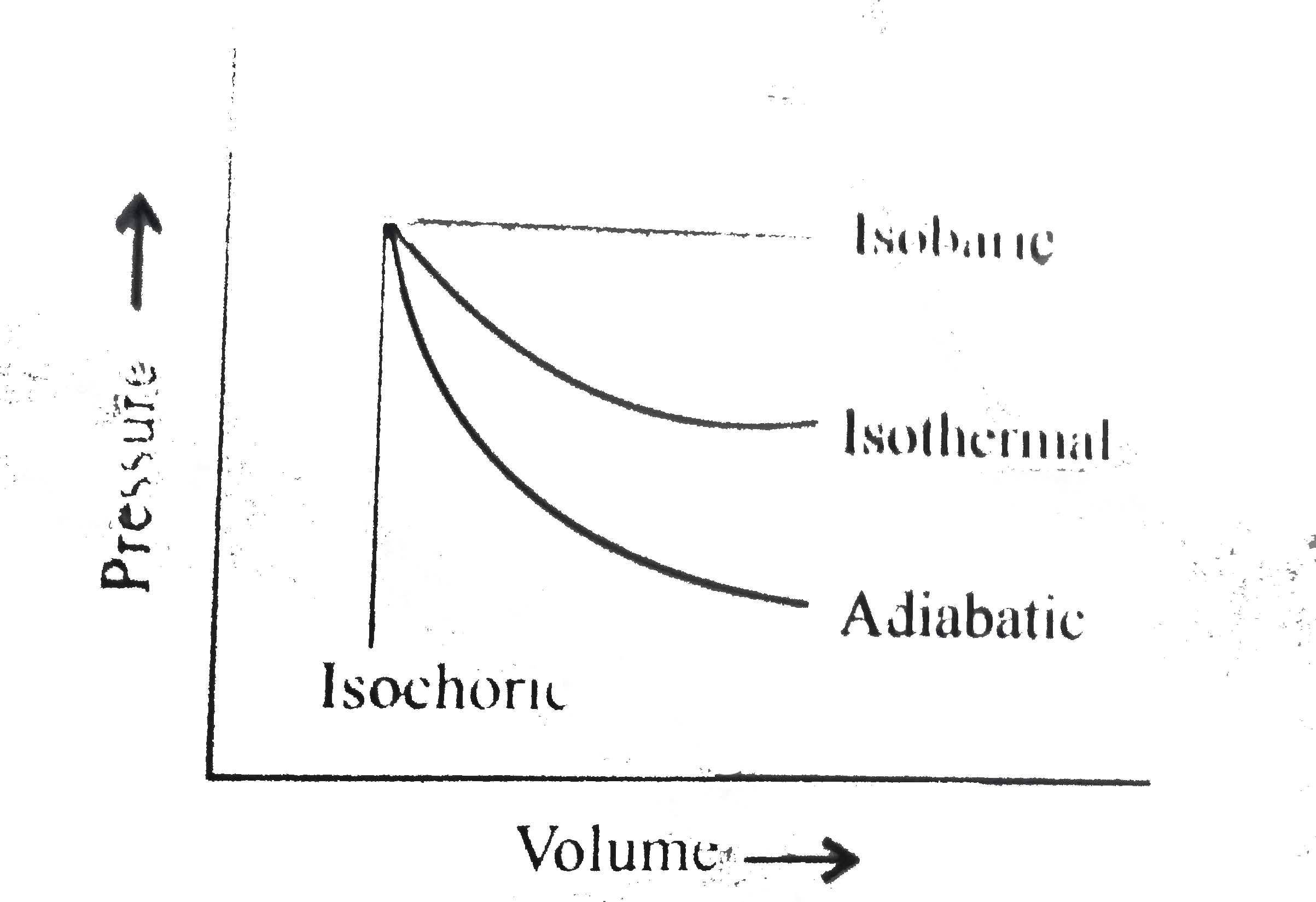
Work is the mole of transference of energy. It has been observed that reversible work done by the system is the maximum obtainable work.
wrev>wirr
The works of isothermal and adiabatic processes are different from each other.
wisothermal reversible=2.303nRTlog10(V2V1)
=2.303nRTlog10(P2P1)
wadiabatic reversible=CV(T1−T2)
The q value and work done in isothermal reversible expansion of one mole of an ideal gas from initial pressure of 1 bar to final pressure of 0.1 bar at constant temperature 273K are:
5.22kJ,−5.22kJ
+6.22kJ,6.22kJ
5.22J,5.22J
−6.22J,−6.22J
w=−2.303nRTlog(P1P2)
=−2.303×1×8.314×273log(10.1)=−5.227kJ
q=−w=+5.227kJ for isothermal process
Similar Questions
Work is the mose of transfrence of energy. If the system involves gaseous substance and there is difference of pressure between system and surroundings, such a work is referred to as pressure - volume work (WPV=−PextΔV). It has been observed that reversible work done by the system is the maximum obtainable work. wrev>wirr
The works of isothermal and adiabatic processes are different from each other. for isothermal reversible proces,
Wisothermal reversible=2.303nRTlog10(V2V1)
Wadiabatic reversible=CV(T1−T2)
Calculate work done when 1 mole of an ideal gas is expended reversibly from 20 L to 40 L at a constant temperature of 300 K.
View SolutionWork is the mose of transfrence of energy. If the system involves gaseous substance and there is difference of pressure between system and surroundings, such a work is referred to as pressure - volume work (W_(PV)= -P_(ext)DeltaV) . It has been observed that reversible work done by the system is the maximum obtainable work. w_(rev) gt w_(ir r) The works of isothermal and adiabatic processes are different from each other. for isothermal reversible proces, W_("isothermal reversible")=2.303 nRT log_(10) (V_(2)/V_(1)) W_("adiabatic reversible")=C_(V) (T_(1)-T_(2)) A thermodynamic system goes in acyclic process as represented in the following P-V diagram: 1. For an ideal gas, consider only P-V work in going from an initial state The net work done during the complete cycle is given by the area:
View SolutionIf W1W2,W3andW4 are work done in isothermal, adiabatic, isobaric and isochoric reversible process, the correct order (for expansion) will be
View SolutionIf the system involves gaseous substance, then pressure volume work Wpv=−PextΔV. It has been observed that,
Wrev>Wir.
Also the work of isothermal & adiabatic processes are different from each other :
Wisothermalreversib≤=2.303nRTlog(v2v1)
Wadiabaticreversib≤=−Cv(T2−T1)
If W1,W2,W3,W4 are work done in isothermal, adiabatic, isobaric & isochoric reversible processes, then correct sequence would be
View SolutionIf the system involves gaseous substance, then pressure volume work W_(pv) = -P_(ext)DeltaV . It has been observed that, W_(rev) gt W_(ir) . Also the work of isothermal & adiabatic processes are different from each other : W_(isothermal reversible) = 2.303 nRT log(v_2/v_1) W_(adiabatic reversible) = -C_v(T_2-T_1) P-V plot for two gases during adiabatic processes are given as follows : Curves A & B should correspond to respectively
View SolutionA sample of ideal gas undergoes isothermal expansion in a reversible manner from volume V1 to volume V2. The initial pressure is P1 and the final pressure is P2. The same sample is then allowed to undergoes reversible expansion under adiabatic conditions from volume V1→V2. The initial pressure being same but final pressure is P3.
The work of expansion in adiabatic process (wadi) is related to work of expansion in isothermal process (wiso) is
View SolutionThe pressure-volume of varies thermodynamic process is shown in graphs:
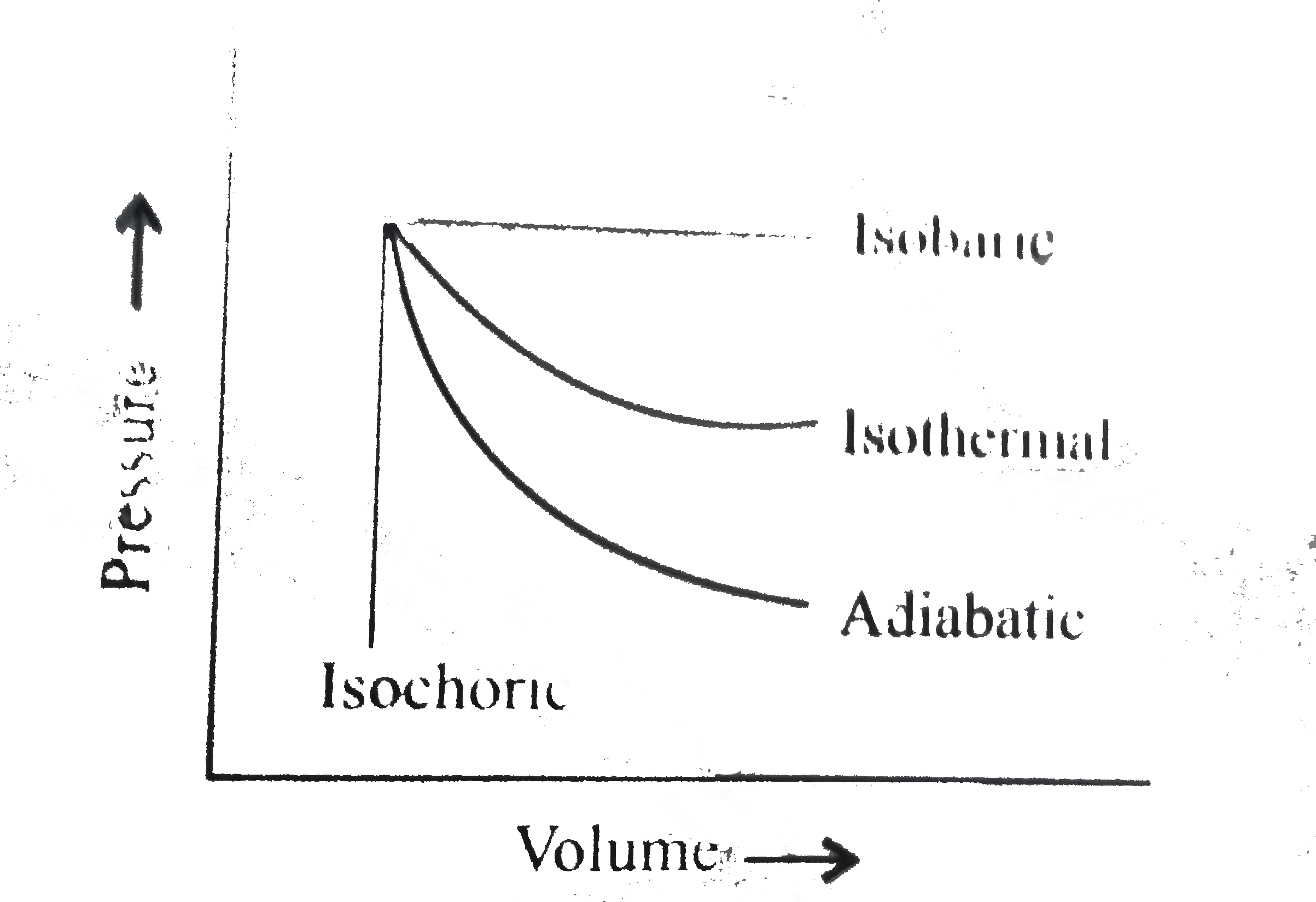
Work is the mole of transference of energy. It has been observed that reversible work done by the system is the maximum obtainable work.
wrev>wirr
The works of isothermal and adiabatic processes are different from each other.
wisothermal reversible=2.303nRTlog10(V2V1)
=2.303nRTlog10(P2P1)
wadiabatic reversible=CV(T1−T2)
If w1,w2,w3 and w4 are work done in isothermal, adiabatic, isobaric, and isochoric reversible processes, respectively then the correct sequence (for expansion) would be
View SolutionThe pressure-volume of varies thermodynamic process is shown in graphs: Work is the mole of transference of energy. It has been observed that reversible work done by the system is the maximum obtainable work. w_(rev) gt w_(irr) The works of isothermal and adiabatic processes are different from each other. w_("isothermal reversible") = 2.303 nRT log_(10) ((V_(2))/(V_(1))) = 2.303 nRT log_(10)((P_(2))/(P_(1))) w_("adiabatic reversible") = C_(V) (T_(1)-T_(2)) A thermodynamic system goes in a cyclic process as represented in the following P -V diagram: The network done during the complete cycle is given by the area
View Solution
