A stable compound has……………………….heat of formation.
Video Solution
|
Answer
Step by step video & image solution for A stable compound has……………………….heat of formation. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Hydrogenation of unsaturated hydrocarbons is an exothermic reaction. Due to hyperconjugation and resonance the stability of unsaturated hydrocarbons increases and the increase in stability is more due to resonance. Compound with same number of π−bonds and more stability has lower heat of hydrogenation.
Heat of formation is defined as the energy evolved when a molecule is formed from its atoms. For isomers the more stable compounds has higher heat of formation.
The order of heat of formation of the following molecules is :
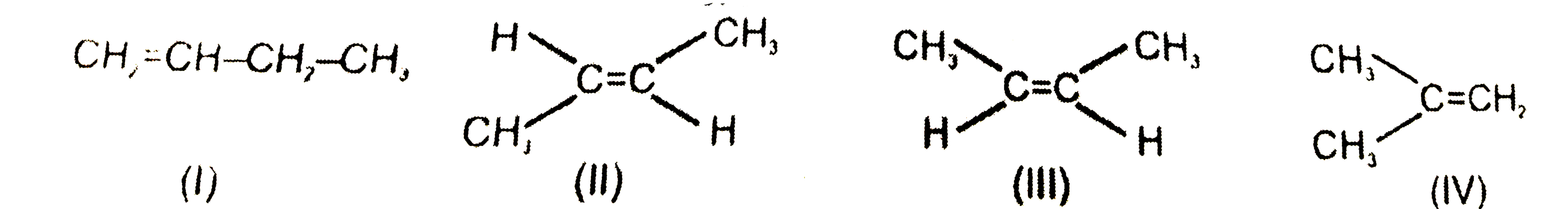 AI>II>III>IVBII>III>IV>ICIV>II>III>IDIV>III>II>I
AI>II>III>IVBII>III>IV>ICIV>II>III>IDIV>III>II>IHydrogenation of unsaturated hydrocarbons is an exothermic reaction. Due to hyperconjugation and resonance the stability of unsaturated hydrocarbons increases and the increase in stability is more due to resonance. Compound with same number of π−bonds and more stability has lower heat of hydrogenation.
Heat of formation is defined as the energy evolved when a molecule is formed from its atoms. For isomers the more stable compounds has higher heat of formation.
The correct heat of hydrogenation order is :
(p) 1,3-Pentadiene
(q) 1,3-Butadiene
(r) 2,3-Dimethyl-1,3-butadiene
(s) PropadieneAp > q > r > sBs > q > p > rCq > s > p > rDs > p > q > rThe most stable compound is
ALiFBLiClCLiBrDLi

