N2(g)+3H2(g)⇔2NH3(g),ΔHɵ=−22.4kJ
The pressure inside the chamber is 100 atm and temperature at 300K
The preparation of ammonia by Haber's process is an exothermic reaction. If the preparation follows the following temperature-pressure relationship for its % yield. Then for temperature T1,T2 and T3 the correct option is:
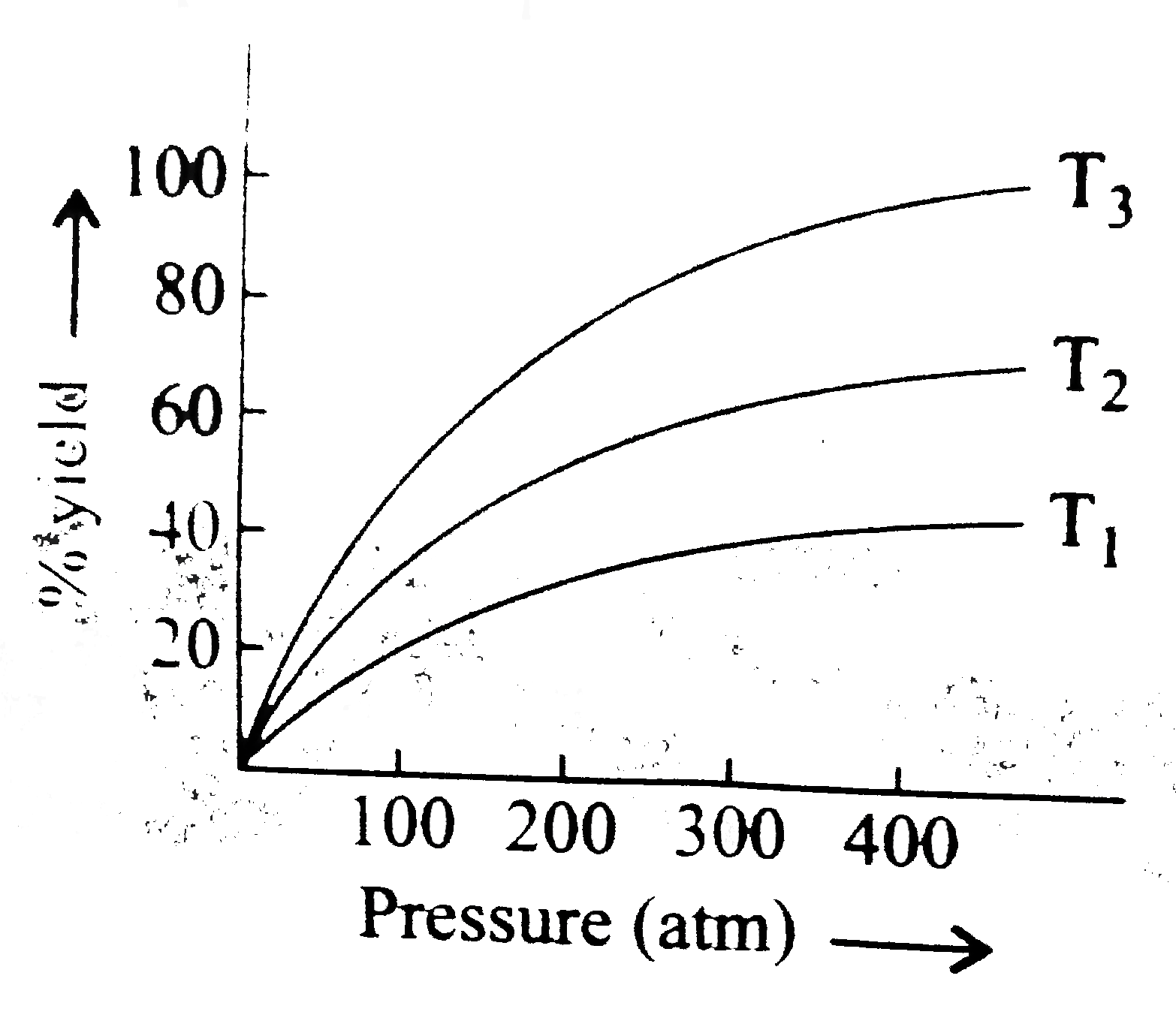
T3>T2>T1
T1>T2>T3
T3<T2<T3
T1=T2=T3
The correct Answer is:B
T1>T2>T3
The % yield will decrease with rise in temperature since it is exothermic.
Similar Questions
N2(g)+3H2(g)⇔2NH3(g),ΔHɵ=−22.4kJ
The pressure inside the chamber is 100 atm and temperature at 300K
If Kp for the given reaction is 1.44×10−5, then the value of Kc will be:
View SolutionN2(g)+3H2(g)⇔2NH3(g),ΔHɵ=−22.4kJ
The pressure inside the chamber is 100 atm and temperature at 300K
If Kp for the reaction is 1.44×10−5, then the value of Kp for the decomposition of NH3
2NH3(g)⇔N2(g)+3H2(g)
will be:
View Solution
Knowledge Check
The preparation of ammonia by Haber's process is an exothermic reaction. If the process follows the following temperature pressure relationship for its % yield, then for temperatures T1,T2,andT3 the correct option is
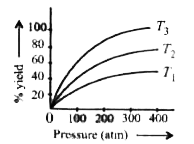 AT3>T2>T1BT1>T2>T3CT1=T2=T3Dnothing could be predicted.
AT3>T2>T1BT1>T2>T3CT1=T2=T3Dnothing could be predicted.N2(g)+3H2(g)⇔2NH3(g),ΔHɵ=−22.4kJ
The pressure inside the chamber is 100 atm and temperature at 300K
On adding catalyst the equilibrium of reaction:AShift in backward directionBShift in forward directionCDoes not affect the equilibriumDCannot predict.The prepation of SO3(g) by reaction SO2(g)+12O2(g)⇔SO3(g) is an exothermic reaction .If the preparation follows the following temperature -pressure relationship for % yield , then for temperatures T1,T2andT3 the correct option is:
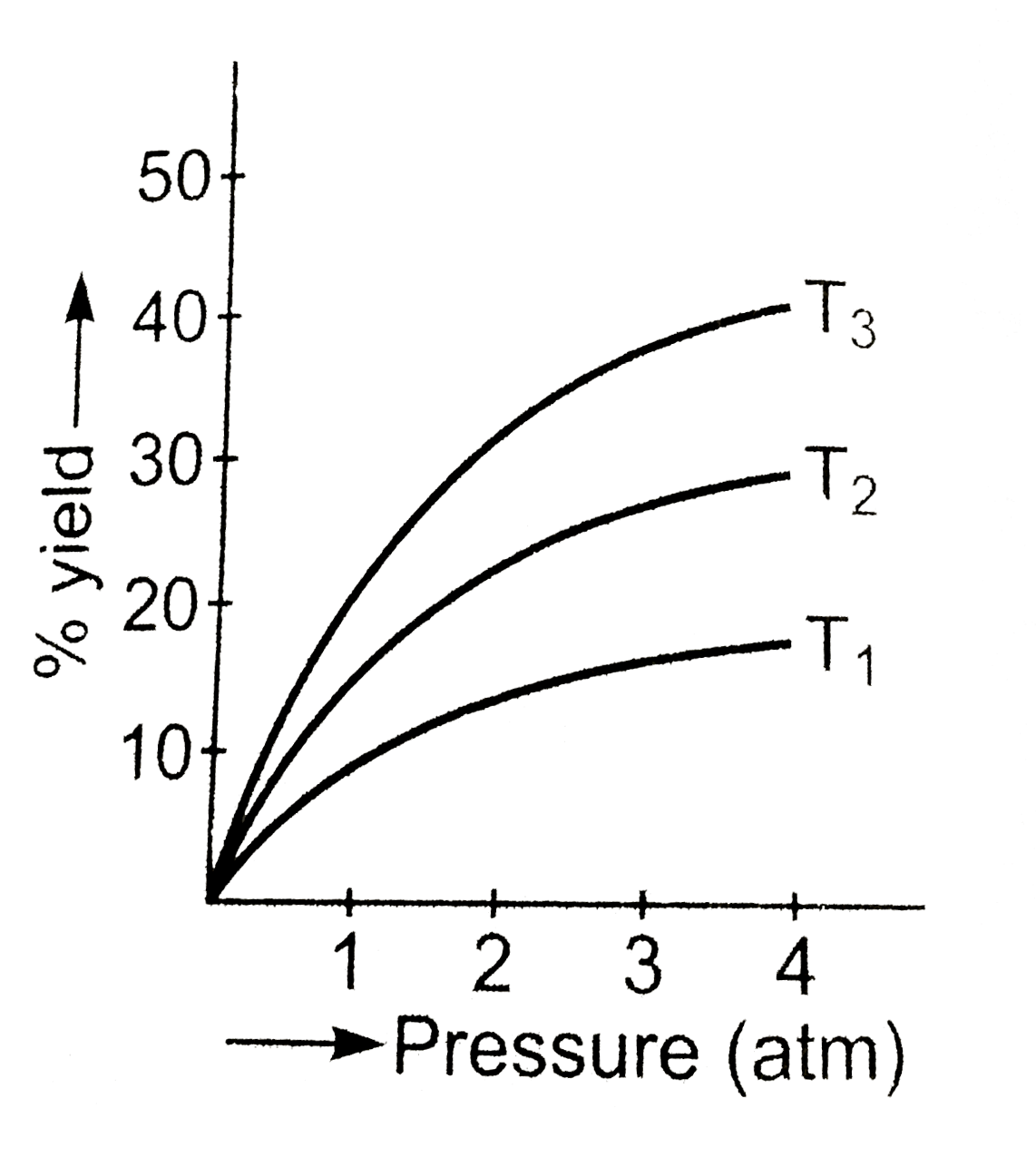 AT3>T2>T1BT1>T2>T3CT1=T2=T3DNothing could be predicated about temperature though given information
AT3>T2>T1BT1>T2>T3CT1=T2=T3DNothing could be predicated about temperature though given information
N2(g)+3H2(g)⇔2NH3(g),ΔHɵ=−22.4kJ
The pressure inside the chamber is 100 atm and temperature at 300K
30LH2(g) and 30LN2(g) were taken for the reaction in Haber's process which yields only 50% of the expected ammonia due to reversibility of the reaction. What will be the composition of reaction mixture under the given condition?
View SolutionN2(g)+3H2(g)Fe+Mo⇐==⇒2NH3(g), Haber's process, Mo is used as:
View SolutionFor the reaction : N2(g)+3H2(g)⇔2NH3(g), ΔH=−ve, the correct statement is :
View SolutionIn reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJ/mole, the yield of ammonia does not increase when
View SolutionFor the chemical reaction
N2(g)+3H2(g)⇔2NH3(g)
The correct option is:
View Solution
