Which of the following will not change the concentration of ammonia in the equilibrium
N2(g)+3H2(g)⇔2NH3(g),ΔH=−xkJ
increase of temperature
increase of volume
decrease of volume
addition of catalyst
The correct Answer is:D
Effect of catalyst is only to alter the speed of the reaction, it does not effect the equilibrium constant and concentration of reactant or product.
Similar Questions
Knowledge Check
For the reaction
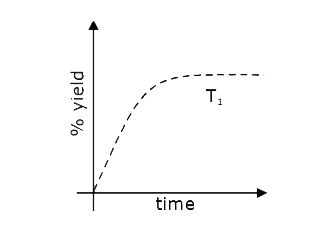
N2(g)+3H2(g)⇔2NH3(g),ΔH=?AΔE+2RTBΔE−2RTCΔE+RTDΔE−RTThe % yield of ammonia as a function of time in the reaction N2(g)+3H2(g)⇔2NH3(g),(g)ΔH<0 at (P,T1) is given below
 A
A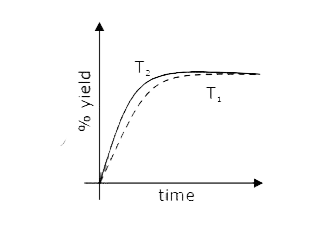 B
B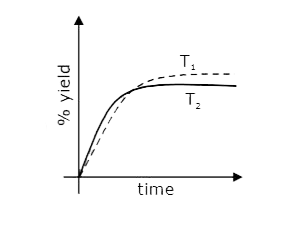 C
C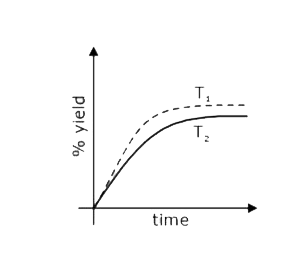 D
D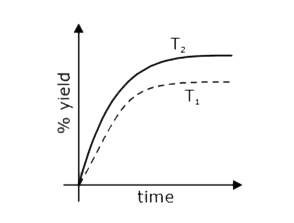
Which of the following is correct for the reaction?
N2(g)+3H2(g)⇔2NH3(g)AKp=KcBKp<KcCKp>KcDPressure is required to predict the correlation
Which of the following is correct for the reaction?
N2(g)+3H2(g)⇔2NH3(g)
View SolutionWhich of the following factors favour the reaction:
N2(g)+3H2(g)⇔2NH3(g)ΔH=-93.6 kJ
View SolutionIn which of the following reactions, the formation of product is favoured by decrease in temperature ?
(1)N2(g)+O2(g)⇔2NO(g),ΔH∘=181
(2)2CO2(g)⇔2CO(g)+O2(g),ΔH∘=566
(3)H2(g)+I2⇔2HI(g),ΔH∘=−9.4
(4)H2(g)+F2(g)⇔2HF(g),ΔH∘=−541
View SolutionThe Habar's process of production of ammonia involves the equilibrium:
N2(g)+3H2(g)⇔2NH2(g)
Assuming ΔH∘ and ΔS∘ for the reaction do not change with temperature, which of the statements is true?
(ΔH∘=−95kJandΔS∘=−198JK−1)
View SolutionWhich of the following is correct for the reaction?
N2(g)+3H2(g)⇔2NH3(g)
View Solution
