The value of Kw, at the physiological temperature 37∘C is 2.4×10−14. What is the pH at the neutral point of water at this temperature where there are equal numbers of H+ and OH− ions?
[H⊕]=√2.4×10−14=1.55×10−7
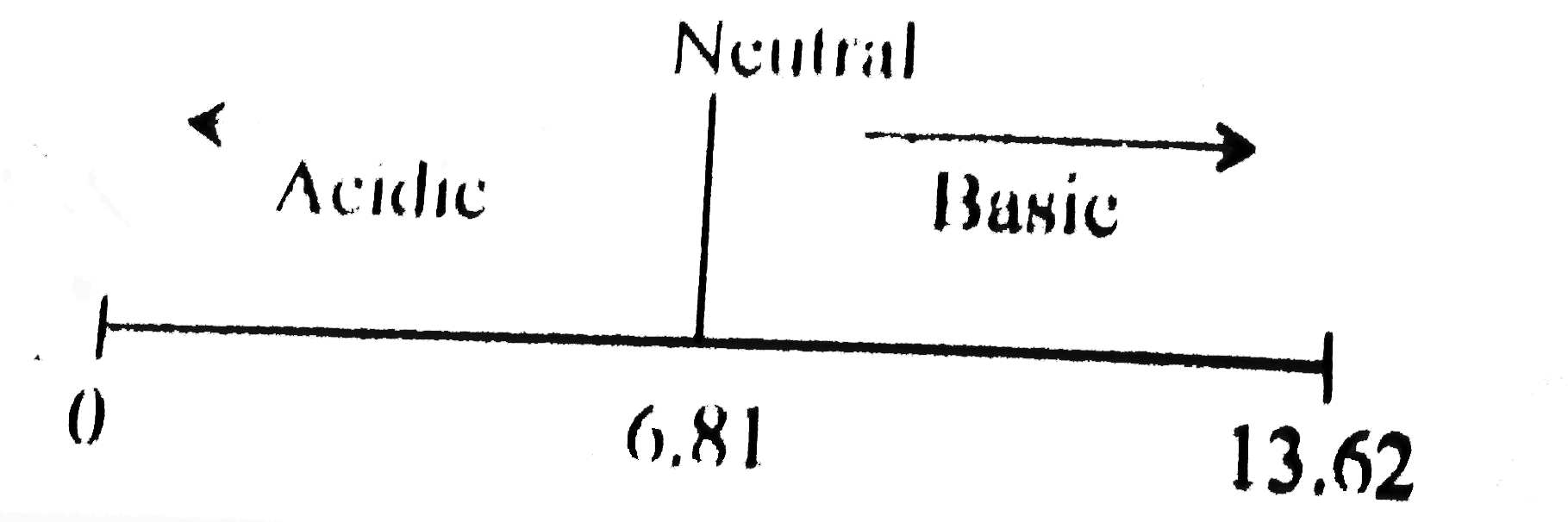
pH=−log(1.55×10−7)=6.81.
Thus, pH scale becomes in between 0 and 13.62.
Similar Questions
The value of Kw, at the physiological temperature 37∘C is 2.4×10−14. What is the pH at the neutral point of water at this temperature where there are equal numbers of H+ and OH− ions?
View SolutionThe ionic product of water at 310 K is 2.7×10−14. What is the pH of neutral water at this temperature ?
View Solution
Knowledge Check
At 20∘C water has Kw=6.807×10−15.What is the pH of pure water at this temperature ?
A6.667B6.920C7.000D7.084Kw for water at 25∘C is equal to 10−14. What is its value at 90∘C-
A10−15B10−17C10−14D10−12At some high temperature the value of Kw is equal to 10−12M2. Calculate the H+ ion concentration of water at same temperature is
A10−12MB10−8MC10−7MD10−6M
Ionic product of water at 310K is 2.7×10−14. What is the pH of netural water at this temperature?
View SolutionThe value of kw is 9.55×10−14 at a certain temperature . Calculate the pH of water at this temperature .
View SolutionThe value of kw is 9.55×10−14 at a certain temperature . Calculate the pH of water at this temperature .
View SolutionThe value of Kw at a certain temperature is 6.25×10−14. Calculate the pH of water.
View SolutionAt certain temperature the H+ ions concentration of water is 4×10−7M then the value of Kw at the same temperature is
View Solution

