Solubility of a solute in water is dependent on temperature as given by
S=Ae−ΔH/RT, where ΔH=heat of solution
Solute+H2O(l)⇔Solution,ΔH=±x
For given solution, variation of log S with temperature is shown graphically. Hence, solution is
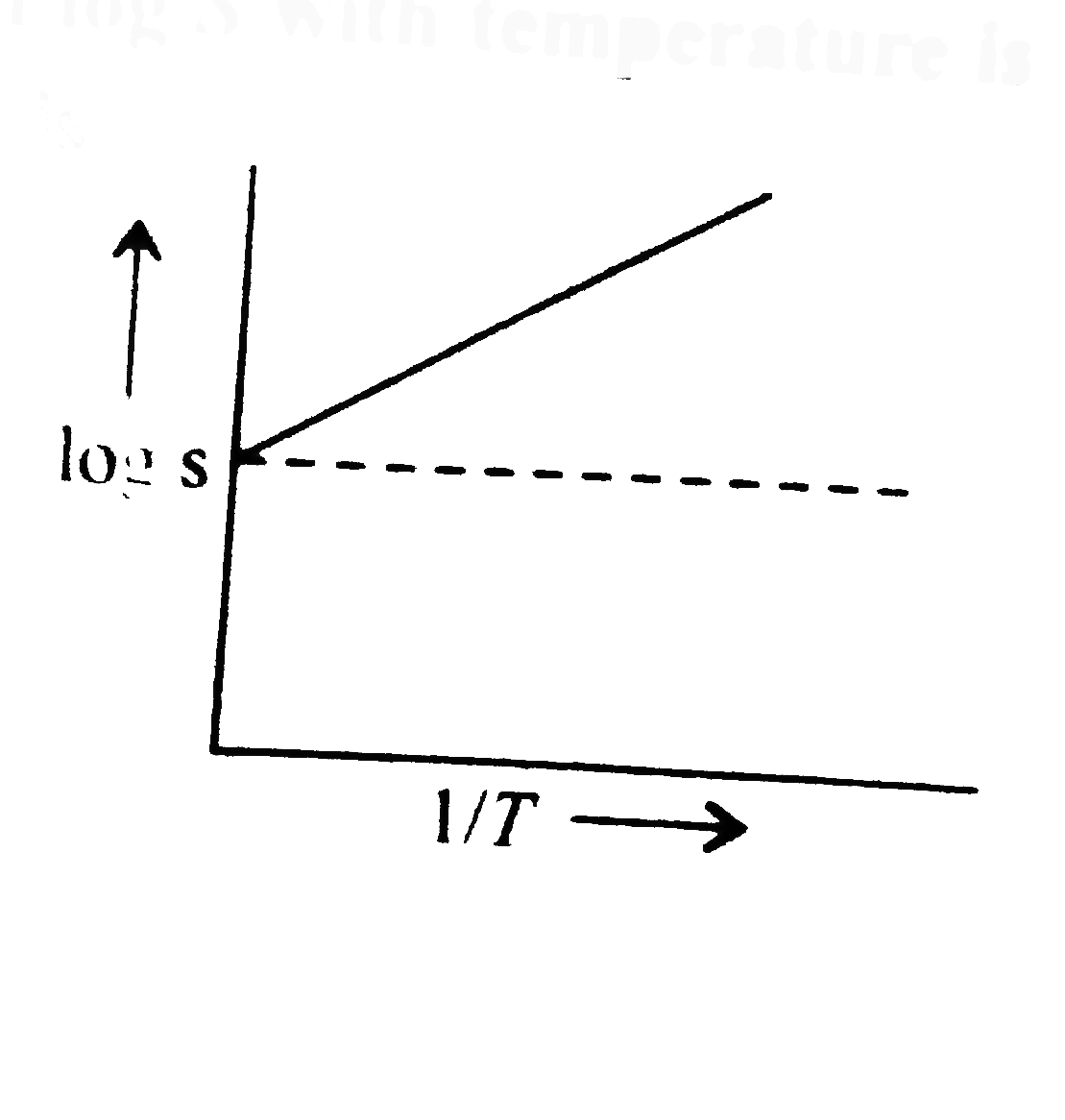
CaO
MgSO4
CuSO4
CuSO4.5H2O
The correct Answer is:D
In Svs1T graph with negative slope.
∴ΔH>0⇒ Salts with water of crystallisation. i.e., CuSO4.5H2O
Similar Questions
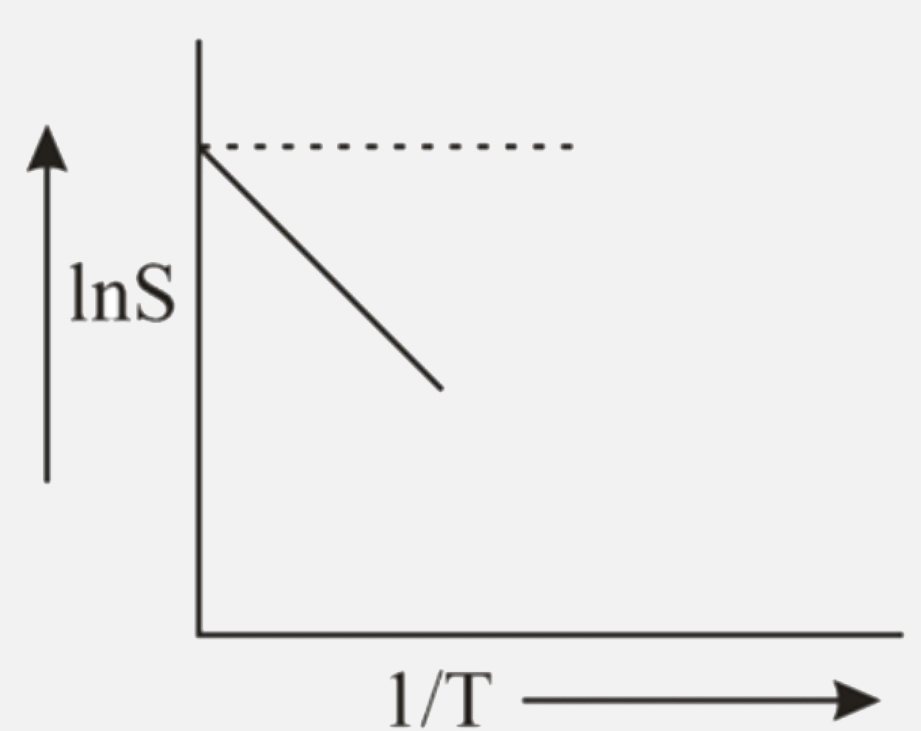
The solubility (S) of solute in water varies with temperature as given by S=Ae−ΔH/RT,ΔH being the enthalpy of solution. For a given solute, variation of lnS with temperature is as shown in figure. The solute is expected to be
View SolutionA solution which cannot dissolve any more solute at a given temperature is called………. Solution
View Solution
Knowledge Check
Solubility (S) of a solution in a solvent (say H2) is dependent on temperature as given by S=Ae−Δ/RT where ΔH is enthalpy of solution, Solute +H2O⇔ solution,ΔH=±x
For a given solution, variation of log S with temperature is shown graphically. Hence, solute is
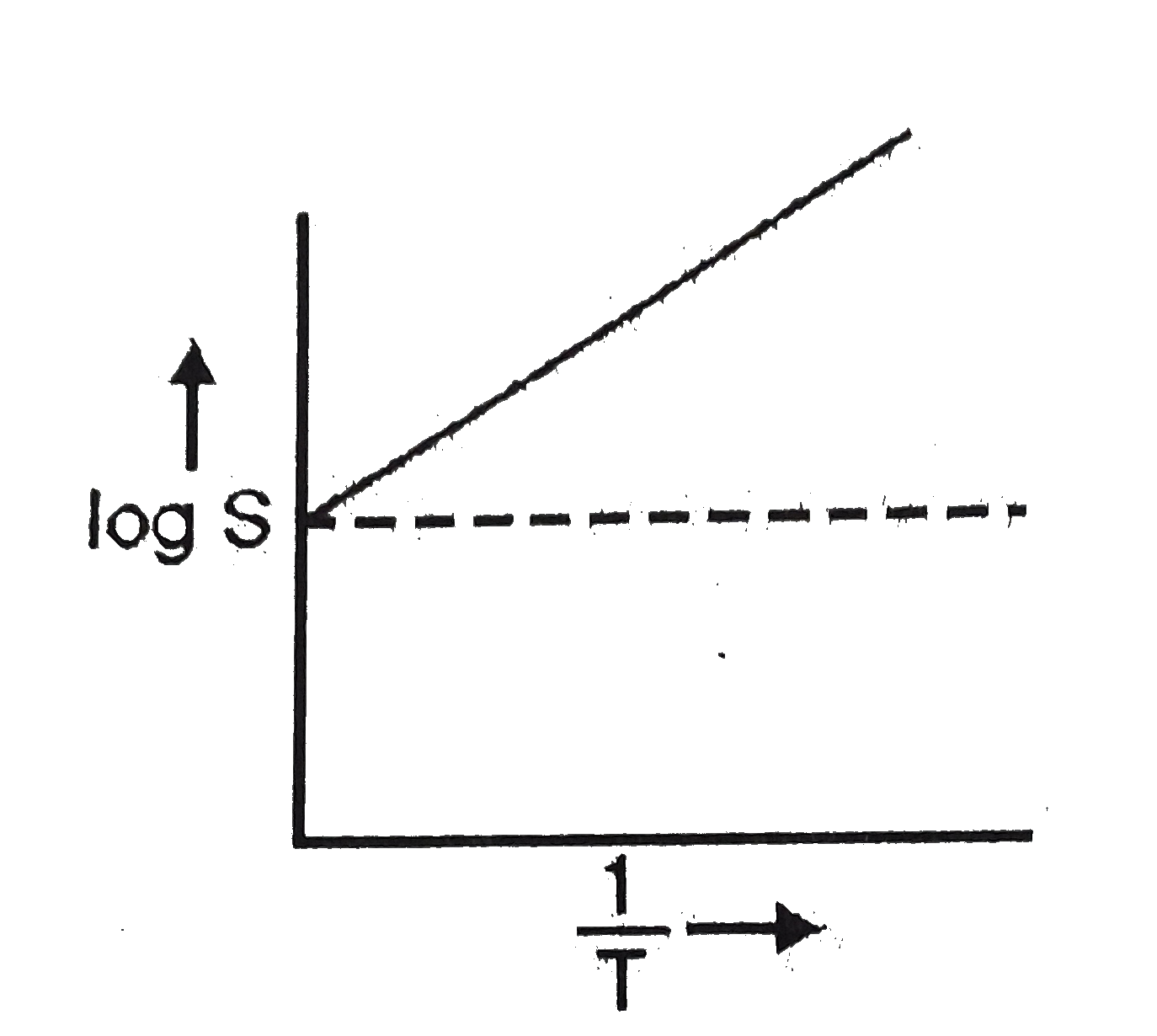 ACuSO4.5H2OBNaClCSucroseDCaO
ACuSO4.5H2OBNaClCSucroseDCaOSolubility of a solute in a solvent ( say H2O ) is dependent on temperature as given by S=Ae−ΔH/RT where ΔH is heat of reaction
Solute +H2O⇔Solution , ΔH=±X
For a given solution variation of logs with temperature is shown graphically. Hence solute isACuSO4.5H2OBNaClCSucroseDCaOOsmotic pressure of a solution at a given temperature
Aincreases with concentrationBdecreases with concentrationCremains sameDinitially increases and then decreases
What happens to the solubility of a gas in a solution with rise in temperature ?
View SolutionSolubility curve of a hydrated salt in water with temperature is given. The curve indicates that the solution process is

View Solution Solubility curve of a hydrated salt in water with temperature is given. The curve indicates that the solution process is :
Solubility curve of a hydrated salt in water with temperature is given. The curve indicates that the solution process is :
View SolutionA solution that has dissolved as much solute as it is capable of dissolving at a given temperature is
View SolutionAt higher temperature, iodoform reaction is given by the dilute solution of
View Solution

