How much of the following are strong electorlytes?
a. NH3 b. NH4CI c. CH3COOH
d. CH3COONa e. HCI f. NaCI
The correct Answer is:D
(b), (d),(e), and (f).
All salts (b,d, and f) soluble hydroxides and the acid (e) are strong electorytes.
Topper's Solved these Questions
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Exercises True/ False
10 Videos
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Archives Multiple Correct
3 Videos
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Exercises Assertion-Reasoning
36 Videos
- HYDROGEN, WATER AND HYDROGEN PEROXIDEBOOK - CENGAGE CHEMISTRYCHAPTER - HYDROGEN, WATER AND HYDROGEN PEROXIDEEXERCISE - Subjective Archive (Subjective)
3 Videos
- ISOMERISMBOOK - CENGAGE CHEMISTRYCHAPTER - ISOMERISMEXERCISE - Assertion-Reasoning Type
1 Video
Similar Questions
How much of the following 0.1M solutions are acidic?
a. NH4CI b. NaOH c. HC2H3O2 d. NaCI
e. NH3+NH4CI f. NH3 g. HCI
h. HCIO4 i.(NH4)2SO4 j. K2SO4
View SolutionWhich of the following may act as buffer? (A) NH4Cl +NH4OH]
(B) CH3COOH+CH3COONa (C) 40ml of 0.1MNH4OH+20ml" .of 0.1MHCl (D) All of these
View Solution
Knowledge Check
Which of the following are isoelectronic specics?
I. CH+3II.NH−2III.NH+4IVNH3AI,III,IVBII,III , IVCI,II IVDI and IIWhich of the following are isoelectronic species?
+CH3
II . NH−2
III . NH+4
IV NH3AI , II and IIIBII ,III and IVCI , II and IVDII and IEnergy profile for the following reaction will be
NH3+CH3−I→CH3−+NH3A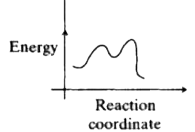 B
B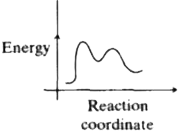 C
C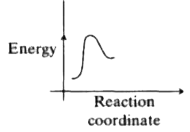 D
D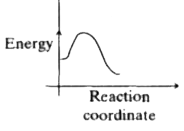
Which of the reagents listed below could be added to water to make 0.1M solutions of each of the following ions?
a. ⊕NH4
b. CH3COOΘ
c. C1Θ
i. NH3 ii. NH4C1
iii. CH3COOH
iv. CH3COONa
v. HC1
vi. NaC1.
View Solution(i) State the formula and name the conjugate base of each of the following species :
(a)H3O+(b)HSO−4(C)NH+4(d)HF(e)CH3COOH(f)CH3NH+3(g)H3PO4(h)H2PO−4.
(ii) State the formula and name the conjugate acid of each of the following species :
(a)OH−(b)HPO2−4(C)H2PO−4(d)CH3NH2(e)CO2−3(f)NH3(g)CH3COO−(h)HS−
View SolutionHow many of the following has hydrogen bonding
(a) NH3 , (b) CH4 , (c) H2O
(d) HI , (e) HF , (f) HCOOH
(g) B(OH)3 , (h) CH3COOH , (i) HCO3−ion
View SolutionWhich of the following are isoelectronic species
I−CH+3,II−NH−2,III−NH+4,IV−NH3
View SolutionWhich of the following increasing order of pH of 0.1 M solution of the compound
(A) HCOONH4
(B) CH3COONH4
( C ) CH3COONa
(D) NH4CI is correct ?
View Solution

