In the structure of diamond, carbon atoms appear at
0,0,0, and 12,12,12
14,14,14, and 12,12,12
0,0,0, and 14,14,14
0,0,0, and 34,34,34
The correct Answer is:A
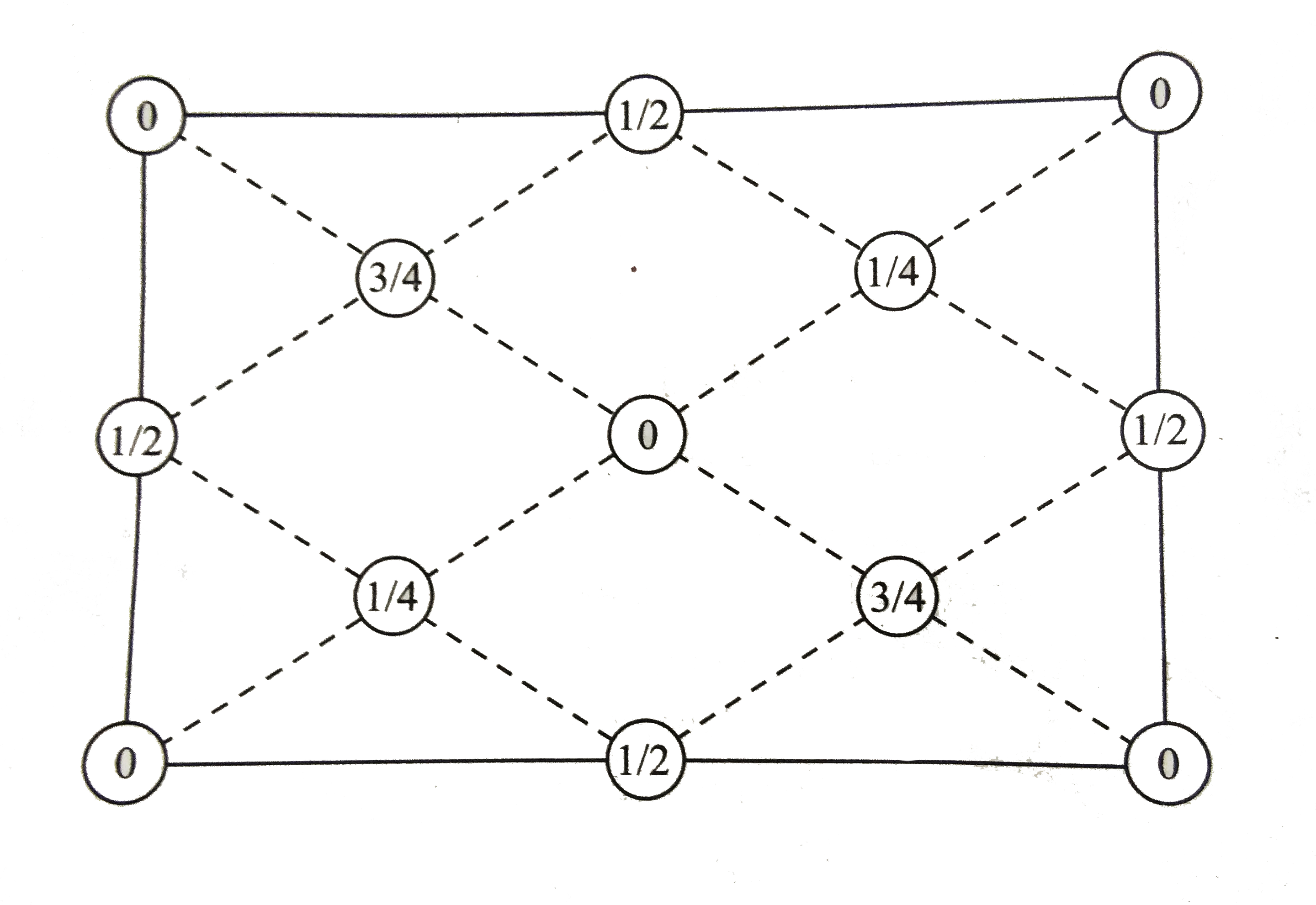
The space lattice of diamond is f. The primitive bassis has two identical atoms at 0,0,0 and 14,14,14 associated with each point of the fcc lattice as shown in the figure.
Similar Questions
Knowledge Check
In the crystal lattice of diamond carbon atoms adopt
AFcc arrangement along with occupancy of tetrahedral holesBFcc arrangement along with occupancy of tetrahedral holesCFcc arrangement along with occupancy of octahedral holeDBcc arrangementIn the crystal lattice of diamond carbon atoms adopt
Afcc arrangement along with occupancy of 50% lertabedral holesBfcc arrangement along with occupancy of 25% lertabedral holesCfcc arrangement along with occupancy of 25% octabedral holesDbcc arrangementCrystal structure of diamond is similar to: -
ANaCl(s)BGraphite(s)CZnS(s)DNa2O(s)
(a) What is diamond ? Of what substance is diamond made?
(b) Describe the structure of diamond. Draw a simple diagram to show the arrangement of carbon atoms in diamond.
(c) Explain why, diamond has a high melting point
(d) State any two uses of diamond.
View SolutionDiamond has a three dimensional structure of C atoms formed by covalent bonds. The structure of diamond has face centred cubic lattice where 50% of the tetrahedral voids are also occupied by carbon atoms. The number of carbon atoms present per unit cell of diamond is_________.
View SolutionA : Number of carbon atoms per unit cell in diamond is 8.
R : The structure of diamond is similar to ZnS.
View SolutionIn diamond structure ,carbon atoms form fcc lattic and 50% tetrahedral voids occupied by acrbon atoms . Evergy carbon atoms is surrounded tetrachedral by four carbon atom with bond length 154 pm . Germanium , silicon and grey tin also crystallise in same way as diamond (NA=6×1023)
The mass of diamond unit cell is:
View SolutionIn diamond structure ,carbon atoms form fcc lattic and 50% tetrahedral voids occupied by acrbon atoms . Evergy carbon atoms is surrounded tetrachedral by four carbon atom with bond length 154 pm . Germanium , silicon and grey tin also crystallise in same way as diamond (NA=6×1023)
The side length of diamond unit cell is (in pm):
View Solution
