Cesium atoms are the largest neturally occurring atoms. The radius of Cs atom is 2.6 Å. The number of moles of Cs atoms to be laid side by side to give a row of Cs atoms 2.5cm long is x×10−17. Find the value of x.
Video Solution
Text Solution
The correct Answer is:8
Statement (a), (b), and (d) are correct.
a. So total score =4+3+1=8
Nearest neighbour of atom 2 are 1 and 3.
Distance between atoms 1 and 2=a
Distance between atoms 2 and 3=√2a

b. Nearest neighbours of atom D are E and F (F atom is in body centre)
Body diagonal (AD)=√34a
Distance DF=12(AD)=√32a

Distance ED=a.
c. Optiion (c) is wrong. ZnS (wurtzite) and ZnS (zinc blende) are polymorphisum.
But in both the compounds, cations Zn2+ ions occupy alternate TVs. ltbr In both compounds, S2− occupied lattice points.
d. Correct statement.
|
Topper's Solved these Questions
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Exercises (Fill In The Blanks)
Explore 15 Videos
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Exercises (True/False)
Explore 19 Videos
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Exercises (Assertion-Reasoning)
Explore 19 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 3 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRYChapter:SOLUTIONSExercise:Ex 2.3 (Objective)
Explore 9 Videos
Similar Questions
Knowledge Check
The number of mole of N - atom in 18.066x1023 nitrogen atoms is
A1 molB2 molC3 molD4 molIf the diameter of carbon atom is 0.15nm, the number of carbon atoms which can be placed side by side is a straight line across length of 10.0cm is
A66.66×107B66.66×108C6.2×109D1.33×107The IE1 of Cs is 376 kj/mole . The number of Cs atoms that can be converted to Cs+ ions by 1J energy is
A1.60×1018B6.023×1018C1.505×1023D3.011×1018
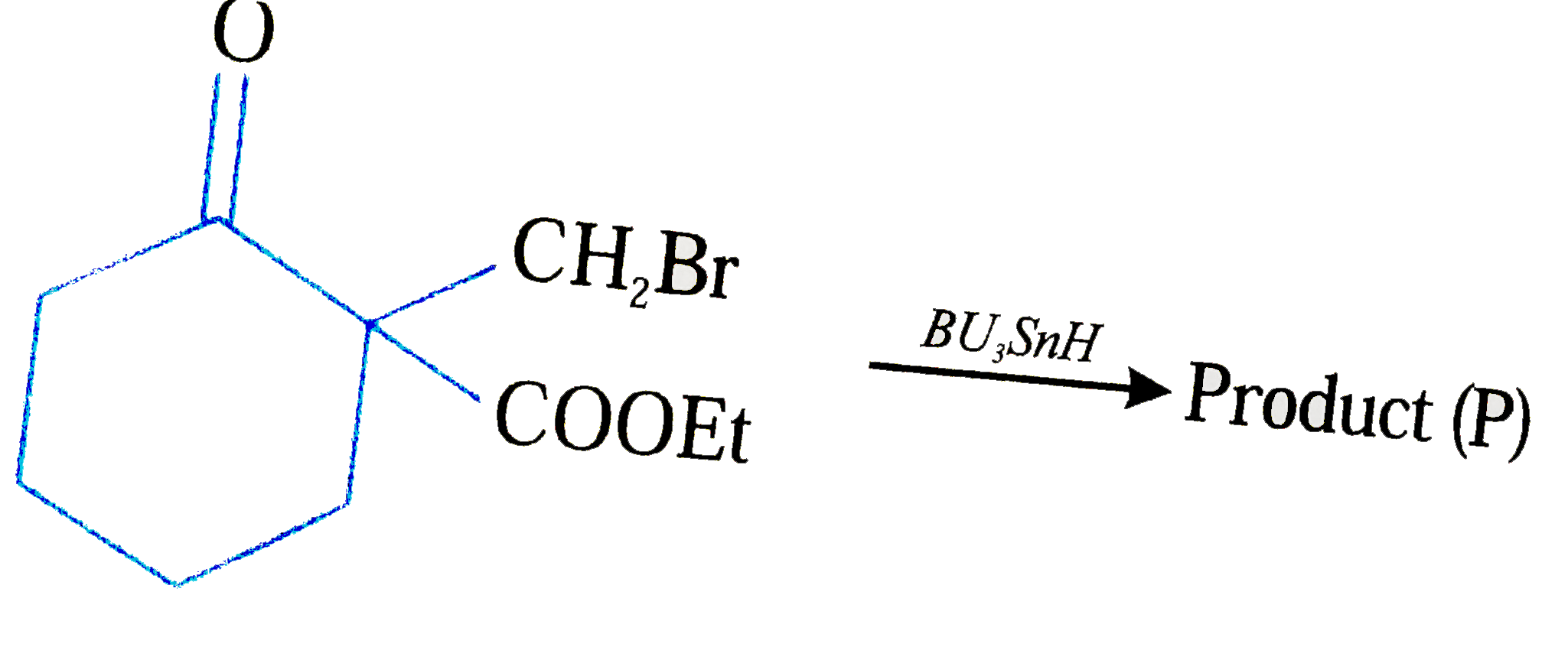
Number of carbon atoms in the product is x. If Y is the number of carbon atoms in the ring of product. Find the value of x-y.
View SolutionIf the diameter of a carbon atom is 0.15nm, calculate the number of carbon atom which can be placed side by side in a straight line length of scale of length 20cm long.
View SolutionThe diameter of zinc atom is 2.6A. Calculate (a) radius of zinc atom in pm and (b) number of atoms present in a length of 1.6cm if the zinc atoms are arranged side by side lengthwise.
View SolutionOne atom of an element x weight 6.643×10−23g. Number of moles of atom in 20 kg is :
View SolutionOne atom of an element x weigh 6.643×10−23 g. Number of moles of atom in 20 kg is :-
View Solution
