In crystals, the state of complete order and of lowest energy is formed at 0∘C.
Video Solution
Text Solution
The correct Answer is:F
Not t zero degree but at zero kelvin.
|
Topper's Solved these Questions
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Exercises (Archives ) Linked Comprehension
Explore 3 Videos
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Exercises (Archives ) Multiple Correct
Explore 2 Videos
- SOLID STATEBook:CENGAGE CHEMISTRYChapter:SOLID STATEExercise:Exercises (Fill In The Blanks)
Explore 15 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 3 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRYChapter:SOLUTIONSExercise:Ex 2.3 (Objective)
Explore 9 Videos
Similar Questions
Knowledge Check
The lowest energy conformer of
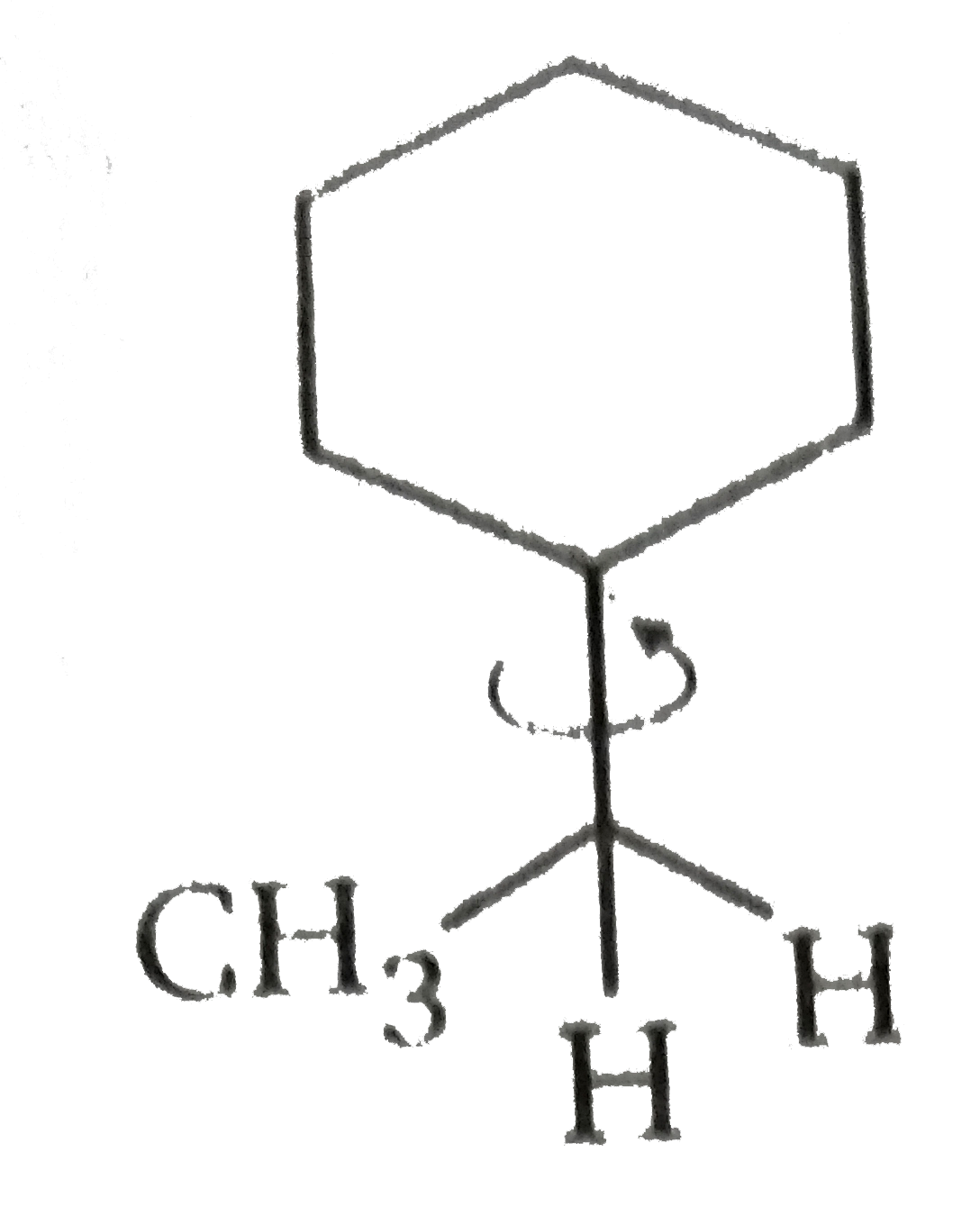 A
A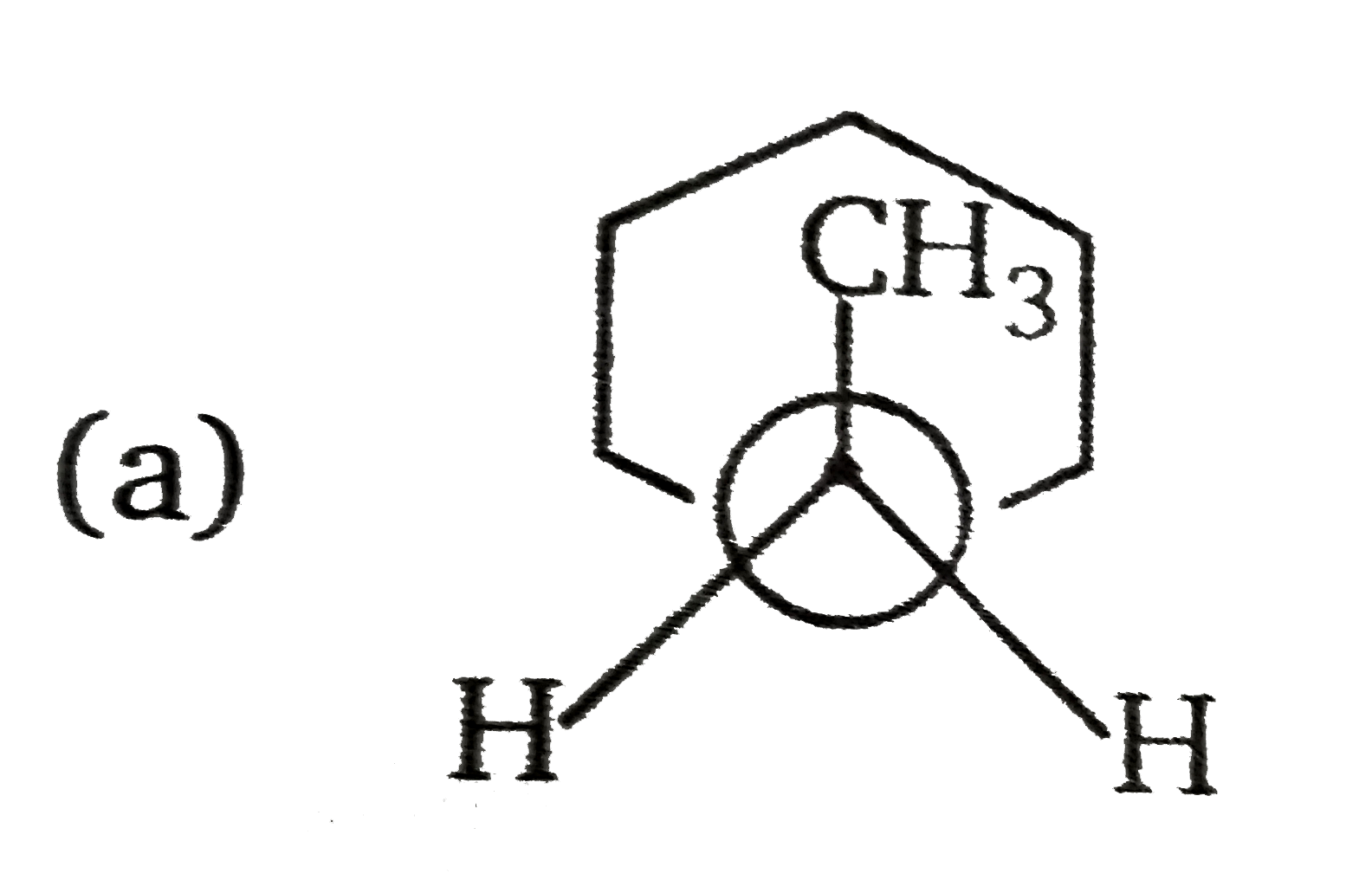 B
B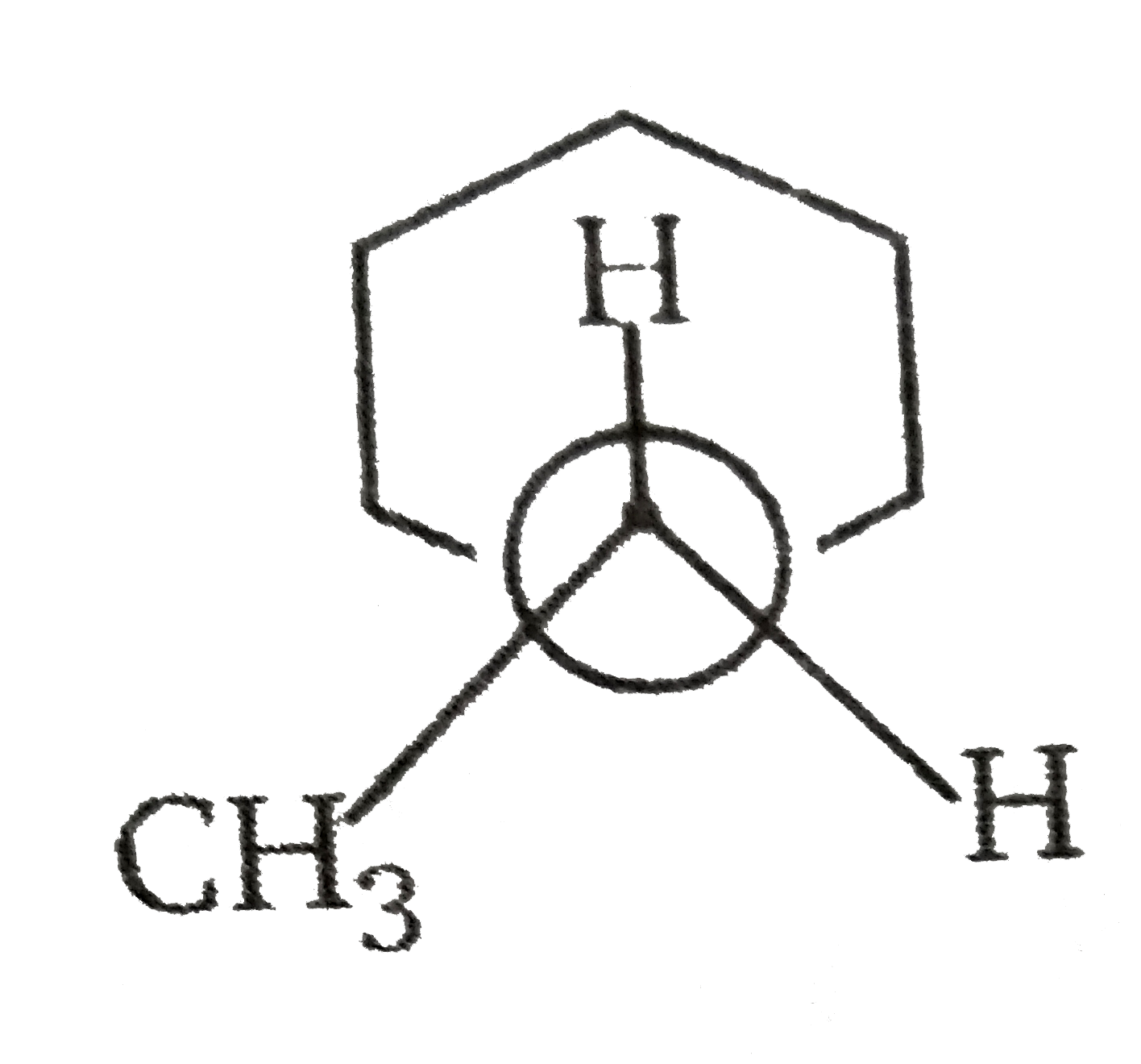 C
C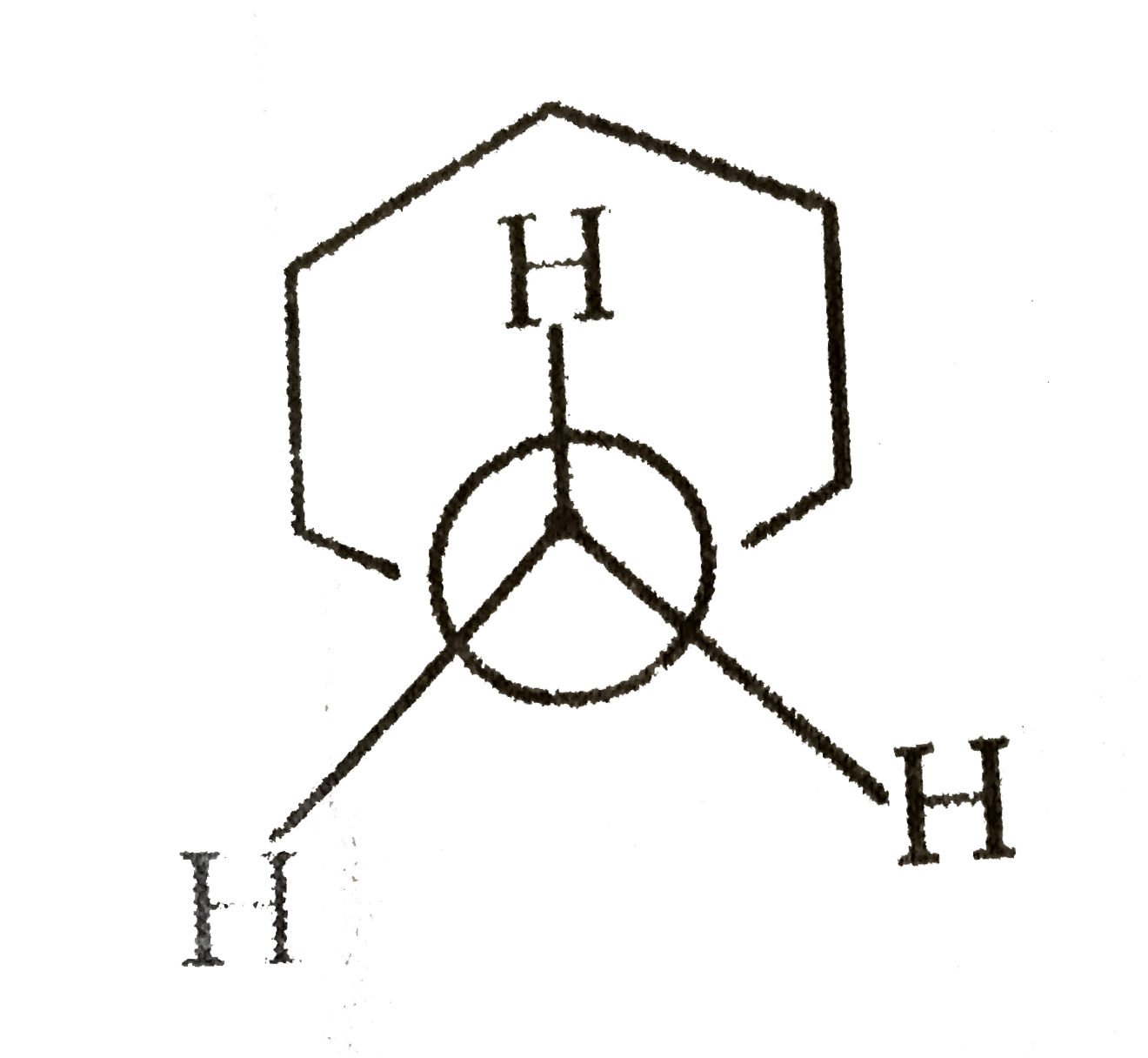 D
D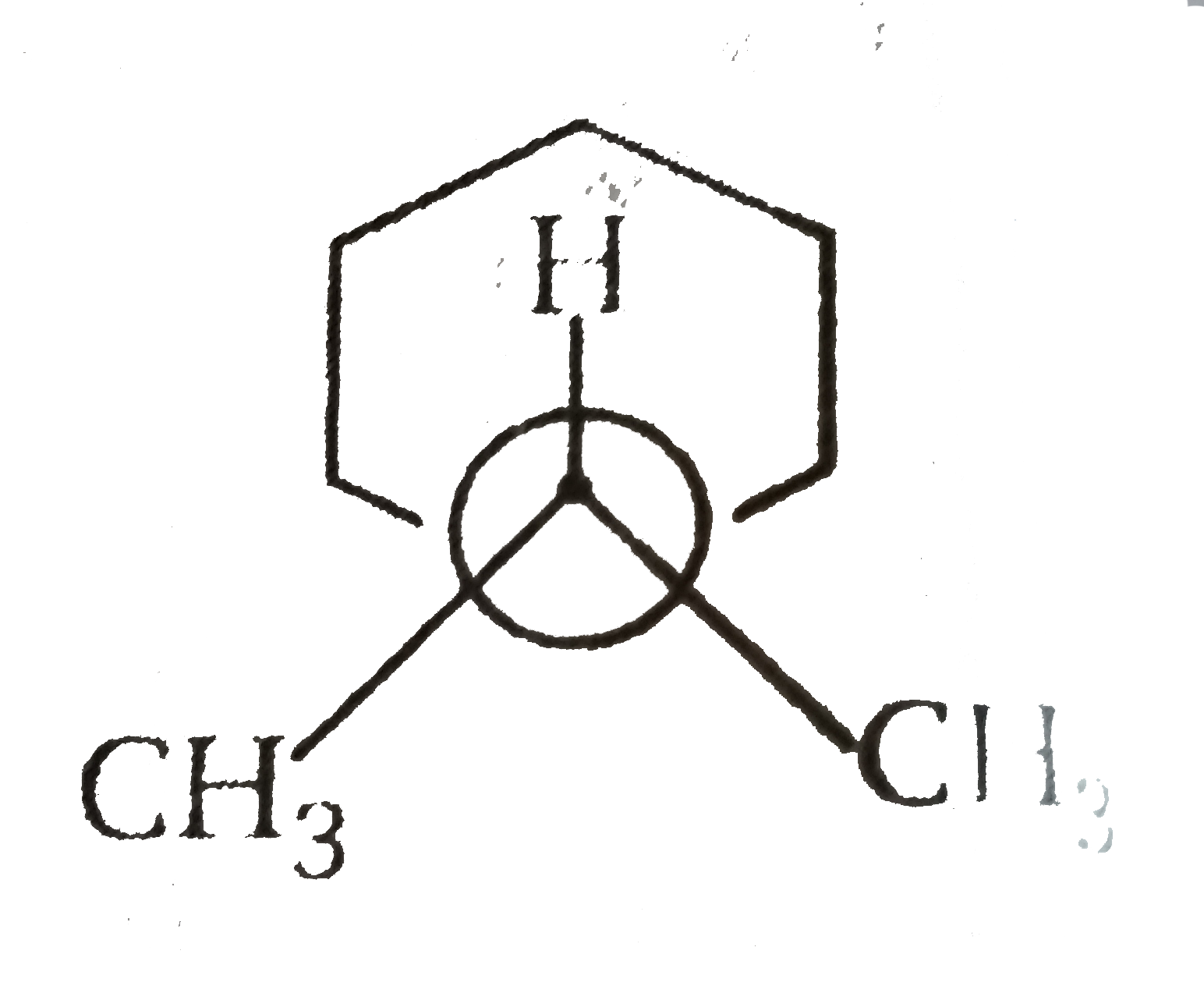
In a crystal, the permitted energy states of electrons are present
Ain the conduction band and the forbidden gapBonly in the forbidden gapCin the valence band and conduction bandDin the forbidden gap and the valence bandThe ionisation energy is lowest for
ANitrogenBOxygenCFluorineDNeon
Name the halogen with lowest ionization energy.
View SolutionState the two forms of energy in which energy is mainly utilised at our homes.
View SolutionWhich state High Court has ordered a complete ban on the sale of whitener in the state in October 2016?
View SolutionHalogen with lowest bond dissociation energy is
View SolutionA proton traps a free electron to form the hydrogen atom of lowest energy level. The wavelength of photon emitted in this process will be (assume initial kinetic energy of the electron to be zero)
View Solution
