The boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
Which among the following is the most volatile and the best antifreeze?
CH3OH
C2H5OH
Glycol
Glycerol
The correct Answer is:A
Step by step video, text & image solution for The boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH_(2)OH-CH_(2)OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH_(3)OH), a fairly volatile liquid that boils only at 65^(@)C, is sometimes used as an antifreeze in automobile radiators. Which among the following is the most volatile and the best antifreeze? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
The boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
620g glycol is added to 4kg water in the radiator of car. What amount of ice will separate out at −6∘C?
View SolutionThe boiling point elevation and the freezing point depression of solutions have a number of practical applications. Ethylene glycol (CH2OH.CH2OH) is used in automobile radiators as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as antifreeze. For the boiling point elevation to occur the solute must be non-volatile, but no such restriction applies to freezing point depression. For example mthanol (CH3OH), a fairly volatile liquid that boils only at 65∘C is sometimes used as antifreeze in automobile radiators.
620g glycol is added to 4kg water in the radiator of a car. What amount of ice will separate out at −6∘C ? Kf=1.86Kkgmol−1.
View Solution
Knowledge Check
The boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
Which of the following is a better reagent for depression in freezing point but not for elevation in boiling point?ACH3OHB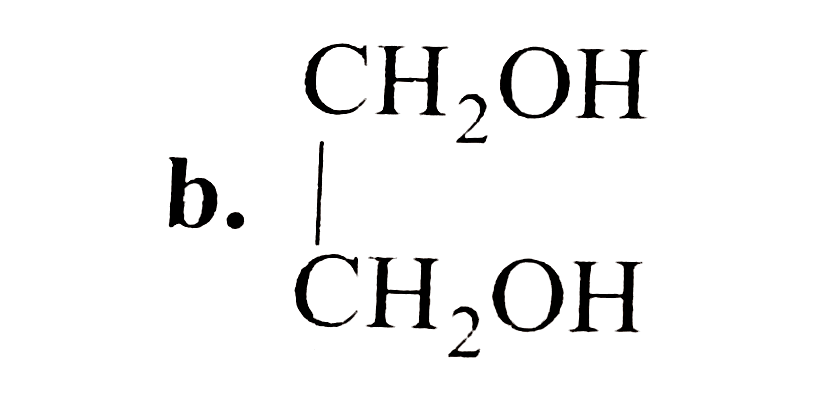 C
C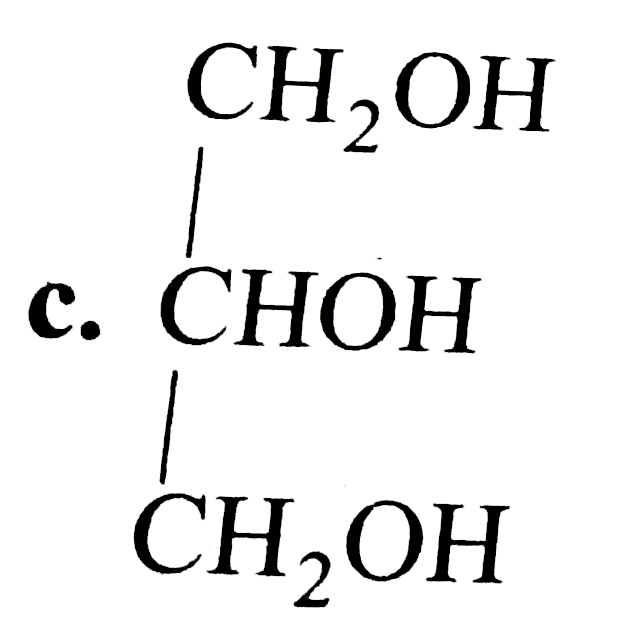 DC6H12O6
DC6H12O6The boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
If the cost of glycerol, glycol, and methanol is same, then the sequence of economy to use these compounds as antifreeze will beAGlycerol gt Glycol gt MethanolBMethanol gt Glycol gt GlycerolCMethanol = Glycol = GlycerolDMethanol gt Glycol < GlycerolThe boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
124g each of the two reagents glycol and glycerol are added in 5kg of water of the radiators in two cars. Which of the following statements is wrong?ABoth will act as antifreeze.BGlycol will be better.CGlycerol is better because its molar mass is greater than glycol.DGlycol is more volatile than glycerol.
The boiling point elevation and the freezing point depression of solutions have a number of practical applications. Ethylene glycol (CH2OH.CH2OH) is used in automobile radiators as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as antifreeze. For the boiling point elevation to occur the solute must be non-volatile, but no such restriction applies to freezing point depression. For example mthanol (CH3OH), a fairly volatile liquid that boils only at 65∘C is sometimes used as antifreeze in automobile radiators.
If cost of glycerol, glycol and methanol are same, then the sequence of economy to use these compounds as antifreeze will be:
View SolutionThe boiling point elevation and the freezing point depression of solutions have a number of practical applications. Ethylene glycol (CH2OH.CH2OH) is used in automobile radiators as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as antifreeze. For the boiling point elevation to occur the solute must be non-volatile, but no such restriction applies to freezing point depression. For example mthanol (CH3OH), a fairly volatile liquid that boils only at 65∘C is sometimes used as antifreeze in automobile radiators.
124g each of the two reagents glycol and glycerol are added in 5kg water of the radiators in the two cars. Which of the following statements is wrong?
View SolutionThe boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
If the cost of glycerol, glycol, and methanol is same, then the sequence of economy to use these compounds as antifreeze will be
View SolutionThe boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
124g each of the two reagents glycol and glycerol are added in 5kg of water of the radiators in two cars. Which of the following statements is wrong?
View SolutionThe boiling point elevation and freezing point depression of solutions have a number of partical applications. Ethylene glycol (CH2OH−CH2OH) is used in automobile radiatiors as an antifreeze because it lowers the freezing point of the coolant. The same substance also helps to prevent the radiator coolant from boiling away by elevating the boiling point. Ethylene glycol has low vapour pressure. We can also use glycerol as an antifreeze. In order for the boiling point elevation to occur, the solute must be non-volatile, but no such restriction applies to freezing point depression. For example, methanol (CH3OH), a fairly volatile liquid that boils only at 65∘C, is sometimes used as an antifreeze in automobile radiators.
620g glycol is added to 4kg water in the radiator of car. What amount of ice will separate out at −6∘C?
View Solution
